* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Managing common contraceptive patient
Race and health wikipedia , lookup
Prenatal development wikipedia , lookup
Reproductive health wikipedia , lookup
Prenatal nutrition wikipedia , lookup
Maternal health wikipedia , lookup
Birth control wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Menstrual cycle wikipedia , lookup
Menstruation wikipedia , lookup
Prenatal testing wikipedia , lookup
Maternal physiological changes in pregnancy wikipedia , lookup
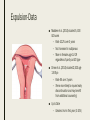
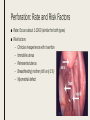
MANAGING COMMON CONTRACEPTIVE PATIENT COMPLAINTS Randee L. Masciola, DNP(c), RN, CNP Women’s Health Nurse Practitioner Coordinator Master’s in Nursing Program Lead Faculty Women’s Health Specialty Track Clinical Instructor The Ohio State University Objectives At the end of the session the participant will: – be able to identify and manage common side effects and complications of intrauterine and other contraception methods – be able to manage unscheduled bleeding in women using hormonal contraception – know where to access up to date information on a variety of common contraceptive side effects and complaints – describe risks and benefits of major contraceptive types, including those that are new to the market Common Contraceptive ComplaintsDefinitions ■ Hormonal – Headache – Nausea – Dizziness – Breast tenderness – Menstrual changes (spotting, BTB, increase/decrease bleeding, amenorrhea) – Mood swings and Depression ■ Mechanical – Decrease sensitivity, cramping, pressure ■ Chemical – Irritation – Allergic reaction Common Hormonal Contraceptive Complaints-Statistics ■ #1 reason women discontinue contraceptive method: Side effects (63%) – According to CDC National Health Statistics (2013) ■ Menstrual Related Side Effects (most common) – COC: Unscheduled spotting reported in 30-50% in first 3 months, and by third pack 70-90% no further unscheduled spotting ■ Headaches (4%) – More frequent with personal/family history and increases with age – Decrease with continued use ■ All other complaints less than 1% and most resolve in three months (Hatcher, 2011) Managing Hormonal Side Effect: Unscheduled Vaginal Spotting/Bleeding ■ R/O more serious cause – Pregnancy, infection, other medications, GI issues ■ MOST COMMON CAUSE: Missed pills ■ Assess for 2-3 cycles ■ Before complete active pills: change progesterone – Formulation or dose ■ Continues to bleed after scheduled bleed: more estrogen – Increase estrogen or decrease progesterone ■ Mid cycle – Try one thing at a time ■ Extended Users – Three day break (Hatcher, 2011) Managing Hormonal Side Effect: Headaches ■ Headache 1st cycle: – only 1 in 3 will have in 2nd cycle – Only 1 in 10 in 3rd cycle ■ R/O other causes – Note associated symptoms – Ask about caffeine, other medications including OTC, drug use – Check BP – Stop hormones while evaluating ■ Not candidate if new onset, worsening of hx of migraines or aura ■ Management option: less estrogen, progesterone only option, extended use Manage common side effects and complications of intrauterine contraception ■ Expulsion ■ Perforation ■ Menstrual Issues ■ Pregnancy ■ Discuss new IUD available ■ Mirena as alternative to hysterectomy with DUB Expulsion-Data ■ Madden et al. (2014) studied 5, 403 IUD users – Rate 10.2% over 3 years – Not increased in nulliparous – More in females age 14-19 regardless of parity od IUD type ■ Dines et al. (2014) studied 2,523 age 13-35yo – Rate 6% over 3 years – Teens more likely to request early discontinuation and may benefit from additional counseling ■ Up to Date – Greatest risk in first year (3-10%) Expulsion-Identify ■ Risk Factors – Prior expulsion – Menorrhagia – Severe dysmenorrhea – Insertion immediately following 2nd trimester AB or postpartum ■ NOT RISK FACTORS – Parity – Age – Length of endometrial cavity ■ Symptoms – Cramping, increase discharge, BTB, postcoital bleeding, dyspareunia, string change (longer or shorter) Expulsion-Management ■ Diagnosis of complete expulsion (in the absence of visual of actual IUD) – Requires ultrasound confirmation – X-ray not in abdomen or pelvis ■ New IUD can be placed immediately assuming criteria of initial placement still met – No evidence to support changing IUD type ■ Higher rate of expulsion – Small studies 14-31% repeat expulsion after 1 year – Risk factors potentially technique or uterine anatomy ■ Repeat placement recommended under sonographic guidance – UNLESS post abortion or postpartum Perforation: Rate and Risk Factors ■ Rate: Occurs about 1-1000 (similar for both types) ■ Risk factors – Clinician inexperience with insertion – Immobile uterus – Retroverted uterus – Breastfeeding mother (still only 1%) – Myometrial defect Perforation: Diagnosis and Management ■ Diagnosis – Shorten strings – Abdomen pain and uterine bleeding ■ Management – Ultrasound (x-ray of ultrasound not available) – ATB’s – Recommend surgical removal – May be offered another under sonogram guidance – Not contraindicated for labor and future vaginal delivery Menstrual Issues ■ Main reason for discontinuation of IUD – Irregular spotting or bleeding first few months – Usually described as intermenstrual (all IUD) – Increase volume (Copper) – Prolonged bleeding (LNg) – Amenorrhea (LNg) ■ Management – NSAIDS – Combined OCP’s 1-3 months – Doxycycline 7-14 days (nonspecific endometritis) – Patient EDUCATION! Should be stable at 6 months Pregnancy ■ Very rare (less than 1%); Risk highest in first year ■ Increase risk for ectopic (2 fold) ■ Miscarriage rate 50% if left in situ, twice as high than if removed ■ No increase birth defects ■ First Trimester – If see strings, remove – If patients wishes to continue pregnancy and can’t see strings: ultrasound guided removal using forceps or hysteroscopy ■ Second trimester – If left in: Increase risk PTL/PTD, fetal loss, infection – If removed: Increase risk ROM, loss, bleeding, fetal trauma if complications – Recommend removal by ultrasound guidance with or without visible strings unless placental/gestational sac involvement – If late second trimester and strings not visible, leave IUD ■ Patient needs counseling! (UptoDate, 2016) New IUS product-Skyla Skyla Mirena ■ 13.5mg Levonorgestrel-releasing ■ 52mg Levonorgestrel-releasing ■ 3 years ■ 5 years ■ Releases about 14mcg of progesterone first 25 days then 5 mcg through expiration ■ Releases about 20mcg of progesterone per day first few years and weans to 10mcg by end Advantages of Skyla ■ Less progesterone ■ Smaller in size ■ Insertion tube has smaller diameter – Skyla 3.8mm (Mirena 4.75mm) – Possibly easier insertion and less painful – Better tolerated for women with smaller uterus (Teens and perimenopausal) ■ FDA approved for nulliparous (more research done on this population) – Mirena package inserts states recommended for woman who have had one child Disadvantage of Skyla ■ NOT FDA approved for menorrhagia – Mirena has this designation ■ More unscheduled bleeding reported than Mirena ■ Potential: only 3 year FDA approval Mirena as alternative to Hysterectomy for DUB ■ DUB leading cause of hysterectomy after conventional treatment failures (50%) – Anemia, quality of life, sexual dysfunction, fatigue ■ FDA approval for menorrhagia (2009) – Reduction in bleeding more than 80% first 3-6months – Reduction in bleeding more than 90% after 12 months ■ Waheed and Malike (2013) – 60 patients who presented with DUB – 30 had Mirena placed ■ – 30 placed on OCP’s ■ ■ 80.7% satisfaction rate (7.7% discontinued) 30% satisfaction rate (50% discontinued) Barrington et al. (1997) – 54 women with heavy periods awaiting hysterectomy in UK – 70% taken off waiting list because of satisfaction after IUD placement Practice Recommendations ■ U.S. Medical Eligibility Criteria (CDC) – Evidence based – Improve safety, accessibility and quality of family planning services – http://www.cdc.gov/reproductivehealth/unintendedpregnancy/pdf/legal_sum mary-chart_english_final_tag508.pdf – http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6205a1.htm ■ ACOG – Collection of committee opinion papers and Practice Bulletins – http://www.acog.org/Womens-Health/Birth-Control-Contraception ■ Hatcher: Contraceptive Technology (2011) Case Study Closing Remarks: Education ■ Explain misconceptions ■ Discuss all possible side effects ■ Tell patients what they need to know even if they don’t ask ■ Compare risks/side effects of using with pregnancy risk ■ Think about STD protection ■ Must know warning signs ■ Discuss Non-contraceptive Benefits ■ Use appropriate language and answer all questions truthfully (Hatcher, 2011) References ■ Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol 2014; 123:585. ■ Barrington JW, Bowen-Simpkins P. The levonorgestrel intrauterine system in the management of menorrhagia. Br J Obstet Gynaecol. 1997; 104: 614-6 ■ Daniels, K., Mosher, WD., & Jones, J. Contraceptives Women Have Ever Used: 1982-2010. National Health Institute Report 2013; 62. ■ Hatcher, RA et al. Contraceptive Technology, 20th edition, Ardent Media, INC. 2011. ■ Jacob NS, Mahnert N, Livingston JB, et al. Comparison of intrauterine device expulsion rates after aspiration abortion or interval insertion. Obstet Gynecol 2014; 123 Suppl 1:10S. ■ Madden T, McNicholas C, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol 2014; 124:718. ■ Speroff L, Darney PD. A Clinical Guide For Contraception, 54th ed, Lippincott Williams & Wilkins, Philadelphia 2011. ■ US Selected Practice Recommendations for Contraceptive Use, 2013. http://www.cdc.gov/mmwr/pdf/rr/rr62e0614.pdf. (Accessed on October 29, 2015). ■ Waheed, S., & Malik, A. (2014). Mirena as an Alternative to Hysterectomy in Cases of Dub. Annals of King Edward Medical University, 19(2).