* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Astronomy Worksheet
Star of Bethlehem wikipedia , lookup
Canis Minor wikipedia , lookup
Dyson sphere wikipedia , lookup
Corona Australis wikipedia , lookup
Corona Borealis wikipedia , lookup
Cygnus (constellation) wikipedia , lookup
Aquarius (constellation) wikipedia , lookup
Future of an expanding universe wikipedia , lookup
Star catalogue wikipedia , lookup
Perseus (constellation) wikipedia , lookup
Type II supernova wikipedia , lookup
Observational astronomy wikipedia , lookup
Stellar kinematics wikipedia , lookup
Timeline of astronomy wikipedia , lookup
Corvus (constellation) wikipedia , lookup
Stellar evolution wikipedia , lookup
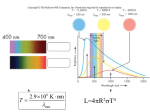
Astronomy Lab Interpreting Stellar Spectra Introduction: From Colors to Curves In this lab you will determine the spectral type of a star by doing spectral analysis. In the process you will review how spectra are used to interpret various stellar parameters. The traditional spectra studied in a high school lab look like a series of colored strips. Researchgrade spectroscopes display this color information as values on a graph. The advantage is that every part of a spectrum is quantified and can be precisely measured. The vertical or y-axis of a spectral graph measures flux, the amount of energy emitted at each wavelength. Like their color counterparts, real stellar spectra are a combination of different types of spectra: continuous, emission and absorption. The continuum is represented by the general trend of the graph. Emission lines are spikes above the continuum, while absorption lines are dips or spikes below the continuum. Each emission or absorption line is the result of electron transitions between energy levels in atoms or molecules. Whether it is emission or absorption just depends on its state: Hot gases = emission, cool gases = absorption. Each molecule and element has its own unique set of these lines, a sort of “fingerprint” that aids its identification. Procedure We will be using a program called Graphical Analysis to examine actual stellar spectra. This is located under the Astronomy class programs menu. Open the program and click OK. Select File/Open. Change the directory to the Students: S drive, then open these folders in order: North/Herrold/Star Spectra. A list of star names will appear. Sign up for the star you have chosen, and write its name here: _________________________ Two very useful tools are located in the Analyze menu. Open the spectrum of your star and activate the first one now: It is called Examine. Notice that a black line appears on the graph. Examine allows you to precisely determine the x, y coordinates of any point along the graph by simply dragging the black line to the desired place. The x position is wavelength in Angstroms, and the y position is flux. Another useful feature is the zoom tool. To magnify a section of the graph, click and drag beginning at the upper left to outline it with a rectangle, then click on the magnifying glass. Directions 1. Open the star’s spectrum. Maximize it according to the directions given in class, and print a copy using the landscape mode. Write your name and the name of the star on the graph. 2. Use Graphical Analysis to identify at least 4 of the major absorption (or emission) lines. Beside each line, write its exact chemical identity, such as Ha Or CaII. Use the Excel spreadsheet of identified spectral lines located in the same folder as the star spectra. Try the common spectral lines section first. 3. Since stars behave as blackbodies, their continuum curves tend to peak at a certain frequency. Find the peak wavelength of your spectrum: ___________Angstroms. Wien’s Law, states that the higher the frequency (or, the shorter the wavelength) of the peak, the hotter the star. Below is a table that shows the color-spectral class-temperature relationships: Class Spectrum Color Temperature O ionized and neutral helium, weakened hydrogen bluish above 31,000 K B neutral helium, stronger hydrogen blue-white 9750-31,000 K A No helium, strongest hydrogen, some ionized metals white 7100-9750 K F weaker hydrogen, many ionized metals yellowish white 5950-7100 K G still weaker hydrogen, ionized and neutral metals yellowish 5250-5950 K K weak hydrogen, many neutral metals orange 3800-5250 K M little or no hydrogen, neutral metals, molecular bands reddish 2200-3800 K L no hydrogen, metallic hydrides, alkali metals red-infrared 1500-2200 K T methane bands infrared 1000 K Temperatures of stars are measured in Kelvins (K). This is an alternative temperature scale to degrees Celsius, and is preferred in most sciences because it eliminates the use of zero in temperature calculations. Wavelengths are measured in Angstroms. One Angstrom (A) = 1 x 10-10m. IN the below equation, “K•A” is a derived unit. Just insert the wavelength of the star in the denominator and solve for its temperature! Use Wien’s Law (below) to calculate the surface temperature of your star: Write the calculated temperature on your graph. Show the set-up your calculation. 4. Use the above chart to estimate the color of your star. Write the color on the graph. 5. Often absorption lines will present as broad, V-shaped notches, in some cases 50 A or more. These are often caused by molecular absorption instead of absorption from a single element, and are known as molecular bands. In addition to gases around stars absorbing energy, Earth’s own atmosphere absorbs some of the frequencies from stars. These telluric absorption bands are well-established and can be seen in every spectrum taken from ground level on Earth. They most often appear near the red end of the spectrum. A set of telluric bands are listed on your spectral lines chart. Locate a telluric band on the spectrum of your star and label it with a T. 6. The spectral class of each star can be determined by using a combination of the chart above (as a starting estimate only!) and the chart on the next page. Read through all of the information that follows- it will help you greatly! Then take some time to study the spectrum of your star, then determine it spectral class. Write the type on the spectrum. 7. Now write a paragraph on the back of the graph that lists the evidence you used to identify the star. You must list 3 pieces of evidence that confirms your spectral class. This should be based primarily on the chemistry of the star, not simply on your temperature calculation. Discuss what lines are present and why they indicate a particular spectral class. You may also use evidence such as the general characteristics of the spectrum. Decoding the Stars The table below characterizes the spectral properties of the different classes: Notes about certain spectral classes: *In general a hot star’s spectrum looks smoother than a cooler star’s spectrum. *In very hot stars (> 10,000 K) most of the Hydrogen gas in the star’s atmosphere will be ionized. Since an ionized Hydrogen atom has no electron it cannot produce any spectral lines, thus the Hydrogen lines are weak in O stars. *A, B and F stars are within the right range of temperatures to energize their Hydrogen gas without ionizing it. Thus the Hydrogen “Balmer” lines are very strong in these stars. At lower temperatures the Hydrogen gas isn’t as easily excited, thus the Balmer lines aren’t as strong in G and K stars, and are barely present in M stars. *Metals are easier to ionize than Hydrogen and Helium and therefore don’t require as high of temperatures, thus spectral lines from ionized metals (e.g., Fe II, Mg II, etc.) are common in stars of moderate temperatures (roughly 5000 to 9000 K). *Metals produce many more spectral lines than Hydrogen and Helium because they have more electrons. In general the cooler the star the more metal lines it will have. CaII 3933, 3968 (known as the “Calcium H and K” lines) is a particularly strong set of lines seen in cooler stars. *In F stars and cooler the Ca II lines are stronger than the Balmer lines. *In the cool G and K stars lines from ionized metals are less abundant and lines from neutral metals are more common. *In the very cool M stars, their atmospheres are cool enough to have molecules that produce wide absorption “bands”, which are much wider than the atomic spectral lines. These absorption bands radically alter the shape of the continuum, to the point where it is not even clear what the continuum really looks like. Finally, these are spectra of real stars, not classroom models! It may seem that your star doesn’t obey the rules. Just as with people, some stars reject the status quo and do their own thing. If you are having real difficulties, consult The Classification of Stars, in the North library. Browse the pages to see if you have one of the renegade types. Extra credit if you can figure out how these strange stars have been classified by astronomers! Sample spectra of some Main Sequence (normal adult) stars. Note: not all stars in this lab are normal adults!!