* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Abstract - School of Engineering and Information Technology
Radio transmitter design wikipedia , lookup
Operational amplifier wikipedia , lookup
Cavity magnetron wikipedia , lookup
Josephson voltage standard wikipedia , lookup
Audio power wikipedia , lookup
Schmitt trigger wikipedia , lookup
Resistive opto-isolator wikipedia , lookup
Valve audio amplifier technical specification wikipedia , lookup
Valve RF amplifier wikipedia , lookup
Voltage regulator wikipedia , lookup
Surge protector wikipedia , lookup
Power MOSFET wikipedia , lookup
Power electronics wikipedia , lookup
Opto-isolator wikipedia , lookup
Current mirror wikipedia , lookup
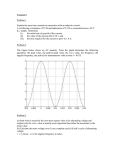
BIO301 Chemostat Group Report Generation of Electricity by Mediatorless Microbial Fuel Cell using Bacteria Source from Activated Sludge Lester Fong, Tony Ho, Ankit Broker, David Hii, Yousif Hanna, Jeremy Hartley & James Atem. Abstract Microbial fuel cells generate electricity by harnessing the electron transport chain of bacteria under controlled conditions, the basic design of most microbial fuel cells consists of an anaerobic anode chamber containing feed source and inoculated with a mixed microbial culture and an anode chamber which contains an oxidizing agent such as dissolved oxygen of ferricyanide. The power output of a microbial fuel cell was measured in terms of a polarisation curve, which shows the relationship between current and voltage over a range of resistances. The polarisation curves were performed for two different methods. Firstly, when the MFC were in batch culture the maximum voltage obtained for the first set of results was 0.272V and the second set of results revealed 0.278V indicating bacteria had increased in health. The columbic efficiency in the batch was recorded at 0.665%, which revealed the bacteria were extremely weak. The first power cure recorded was at 0.0035 mW at a current of 0.0372 mA which was rather low. Furthermore the second power curve showed a maximal power output of 0.0089 mW at a current of 0.063 mA revealing the bacteria were healthier as the maximal power was higher and at a lower resistance compared to the previous one. Resistance kept decreasing when the power was at its maximum. Finally when the MFC were introduced in a continuous culture (chemostat), the maximum voltage obtained from both sets of chemostat curves was 0.556V, which showed an increase in the voltage when compared to the batch culture (0.278V & 0.272V). 1 Introduction Microbial fuel cells (MFC’s) generate electricity by harnessing the electron transport chain of bacteria under controlled conditions (Mohan et al., 2007). They have potential to generate electricity from a wide variety of organic wastes while oxidising the wastes to less harmful forms (Moon et al., 2006, Ieropoulos et al., 2005, Liu et al., 2004). Research into developing efficient MFC’s remains a very current field, with both engineering and biological challenges yet to be met (Moon et al., 2006, Mohan et al., 2007). The basic design of most microbial fuel cells consists of an anaerobic anode chamber containing a feed source and inoculated with a mixed microbial culture, and an anode chamber which contains an oxidizing agent such as dissolved oxygen of ferricyanide (Mohan et al., 2007). Being deprived of a direct electron acceptor for respiration, the bacteria in the anode chamber donate electrons to the anode, which are then transferred via a conductor to the cathode, where reduction occurs (Ieropoulos et al., 2005). Charge balance is maintained by migration of H+ across a proton exchange membrane (Mohan et al., 2007). 2 Figure 1: Schematic representation of a mediatorless MFC operating in batch mode using acetate as an electron donor and ferricyanide as an electron acceptor. MFC’s may be broadly classified into two categories depending on the means of electron transfer between the bacteria and the anode. Mediated fuel cells contain an artificial mediator in the anode chamber (Liu et al., 2004). Bacteria transfer electrons to the mediator in solution, which is then regenerated at the anode (Ieropoulos et al., 2005, Liu et al., 2004). This mechanism of electron transfer has several disadvantages relating to the cost and toxicity of artificial mediators (Ieropoulos et al., 2005, Mohan et al., 2007, Liu et al., 2004). A second category of MFC’s does not contain an artificial mediator, but relies on natural electron transfer processes of the bacteria. While these processes are as yet poorly understood, they are thought to include direct electron transfer by membrane bound enzymes as well as synthesesis of natural mediators (Ieropoulos et al., 2005, Stams et al., 2006, Liu et al., 2004). 3 Because not all substrate is completely oxidised, with some mass necessarily being used for biosynthesis, then not all high energy electrons supplied in the substrate are transferred to the cathode and available to do work. The percentage of electrons which are transferred is expressed in terms of columbic efficiency, which is essentially a percentage ratio of the number of electrons supplied against the number of electrons transferred. This parameter is a useful measure of the overall efficiency of the MFC (Liu et al., 2004, Min and Logan, 2004, Williams, 1966). The power output of a MFC is also a useful quantity to measure. This is measured in terms of a polarisation curve, which shows the relationship between current and voltage over a range of resistances(Mohan et al., 2007, Larminie and Dicks, 2003). By the relationships V=IR and P=IV, where V is voltage, I is current, R is resistance, and P is power, then observations of current, voltage and resistance can be manipulated to give information about power output (Atkins and de Paula, 2006, Serway and Faughn, 2003). The aim of this experiment shall be to create a MFC which is capable of degrading wastewater to produce electricity, and to investigate it’s performance under both batch and continuous flow conditions. Materials and Methods Microbial Fuel cell setup The fuel cell consisted of two chambers (each 500 mL), an anode chamber and a cathode chamber, which were separated by a proton exchange membrane between solutions, and a conductor between the electrodes. The anode consisted of a carbon sponge connected to a platinum wire, while the cathode was composed of platinum foil.(Figure 9).The anode chamber was inoculated with activated sludge and kept under anaerobic conditions, while the cathode chamber was filled with 500mL Potassium Ferricyanide and kept under aerobic conditions. (Figure 10).Both chambers were stirred magnetically and maintained in a water bath at 30oC. The circuit between 4 electrodes was closed to allow electron transfer. (Figure 2). The solution in the cathode chamber was changed when the fading colour of the solution indicated near complete reduction of the Ferricyanide. Operation Batch mode: Synthetic wastewater was prepared with acetate as a carbon source and electron donor. At this stage of the experiment the acetate was kept as a separate solution to limit microbial contamination. The acetate solution was prepared to a concentration of 1.00 M , and the composition of the other component of the synthetic wastewater is given below): 1L Synthetic wastewater consists 20mL of sodium acetate 1.0M, 1.25mL trace elements, 480mg NaHCO3, 95.5mg NH4Cl, 10.5 K2HPO4, 5.25 KH2PO4, 63.1 CaCl2.2H20, and 19.2 MgSO4.7H20 (Ghangrekar and Shinde, 2006). Chemostat mode: Synthetic was supplied to the anode chamber at a rate of 50 mL per day via a peristaltic pump running on a timer cycle of 288 minutes off, 1 minute on. The substrate reservoir contained a single part AWW including diluted acetate component. It was kept in an insulated icebox to reduce microbial activity, and stirred magnetically and intermittently on a timer cycle of 0.5 minutes every 70 minutes to increase homogeneity but minimise aeration. (Figure 11). Monitoring of voltage Voltmeter was used to measure the potential against 1000ohm and the values were recorded down periodically. Polarization curves Polarisation curves were used as a means of determining the capacity of the bacterial culture. A substrate saturated culture was used, and voltage was measured at a series of stepwise increasing resistances until no voltage was measurable between the electrodes. Using the relationship V=IR, then current was thus calculated at each point of the curve, allowing power to also be calculated by the relationship P=IV 5 Methods for obtaining polarization curve The resistor was disconnected and the potential was allowed to build up to a point where no further increment of voltage can be observed. This was followed by observation of voltage increment using voltmeter until it reaches the maximum potential point. Then, the resistor was connected and ensured that the connection is a close circuit system as illustrated below: Figure 2: Closed circuit system of microbial fuel cell The potential values were recorded while varying the resistance from the highest to the lowest resistance at time intervals of 5 minutes. (Note: The voltage values must be taken only when the pseudo-steady-state conditions have been established).Graphs of cell cottage (V) versus current (mA) and Power (mW) versus current (mA) were plotted by referring to the recorder values. Methods for obtaining Columbic efficiency in batch culture The resistor was connected to the anode and cathode chambers to establish a closed circuit system (As illustrated in figure 4). After that, the potential was measured using a voltmeter. the microbial fuel cell was left to run overnight for the voltage reading of 6 the potential to reach stabilization. Then, 1mL sodium acetate 1.0M was added to the anode chamber using Terumu needle (0.65 x 32mm) with syringe. After addition, the voltage reading from the voltage meter was recorded at an interval of 2 hours (Take note of the highest possible voltage reading before the curve start to drop – refer results) and eventually stabilize. Stirring speed When Speed During batch culture 220rpm 1st polarization curve (batch mode) 360rpm 2nd polarization curve (batch mode) 360rpm 3rd polarization curve (chemostat) 200rpm 4rd polarization curve (chemostat) 200rpm Temperature The temperature of the water in aquarium was maintained at around 30°C (this is to ensure suitable temperature for the bacteria to grow in the anode chamber). The ice in the Esky Box was changed 2 times per day (this prevents contamination due to growth of other microorganisms) pH pH of the anode chamber was maintained at around pH6.5 – pH7.0. 7 Results Initially, results were taken when the MFC was in a batch culture. Figure 3: Batch polarization curves. From such calculations, a variety of curves were produced. The polarisation curves produced showed the effects of voltage on the current (Fig 3). Such a graph shows the lowering of the potential of an electrode from equilibrium, which is caused by the passing of an electric current. On the other hand, a power curve illustrates the highest amount of power that the bacteria could produce, showing that the higher the maximum power, the ‘stronger’ the bacteria were. The other main reason for a power curve is that, not only does it show that maximum potential output of the MFC but also at what resistant. For the first polarisation curve, the higher the current, the greater the drop was in the voltage (maximal velocity of 0.2724V) (Fig 3). Although it was not really visible from the graph (refer to table 1), at the beginning (peak voltage), there was a slight increase and then decrease in voltage. This area is where activation losses are dominant (voltage area between 0.2725V and 0.2704V). Furthermore, ohmic losses were dominant when the voltage was between 0.2704V and 0.2664V. When there was 8 a rapid, linear decrease in the voltage, at higher currents (between 0.2664V and about 0.0103V), concentration loss (mass transport effect) was overriding. The second polarisation curve was produced according to results that were taken three days after the initial set of results (Fig 3). As seen in the previous results, the higher the current, the greater the drop was in the voltage (Fig 3; Table 2). A higher maximal voltage was achieved, which was 0.278V compared to 0.2724V. Activation losses did not really dominate at all in this polarisation curve. If there was any domination, it would have been between a voltage of 0.278V and 0.270V. Ohmic losses were dominant when the voltage was between 0.270V and 0.2438V. When there was a linear decrease in voltage, concentration loss (mass transport effect) was dominant at a voltage between 0.2438V and 0.0055V. To compare with the first polarisation curve, the second one slightly shifted to the right, showing higher voltages at higher currents (0.097mA (0.0145V) compared to the first curve, which had a current of 0.097mA and a voltage of 0.0032V). Figure 4: Batch power curves. The first power curve showed that the power of the bacteria had a maximal output potential of 0.0035mW, which was rather low (closed circuit only, as open circuit showed no power) (Fig 4). This power was achieved at a current of 0.03728mA. At 9 maximal power, also, the resistance was 2500 ohms. The maximal volumetric power equals power (W) divided by metres cubed (volume (L) of anode) (Table 1). This occurs at the maximal output potential and in this part of the experiment, it equalled 1.39x10-5 W/m3, which was also a very low number. The reason for the drop in power, after it maximises, is due to an increase in ohmic loss and electrode over potentials (short circuit situation). The maximal power output achieved for the second power curve was higher than the previous curve (a slight shift to the right) (Fig 4). The second power curve showed a maximal power output of 0.0089mW at a current of 0.063mA. At the maximal power, the resistance was 2200 ohms, showing that the bacteria had become slightly more ‘healthier’, as the maximal power was higher and at a lower resistance (compared to the previous power results). The maximal volumetric power was 3.54x10-5 W/m3, which was also higher than the previous result of 1.39x10-5 W/m3, emphasising vast bacterial improvement. In batch mode, the columbic efficiency curve was plotted with current (mA) versus time (h). It represents the total amount of time required for the bacteria in the MFC to completely metabolise 1mL of 1M acetate. Calculated values for the graph can be seen in Table 5 of the appendix. 10 Added 1mL Acetate (1M) Figure 5: Current as a function of time (determines coulombic efficiency) This part of the experiment was carried out three days after the second set of batch results were achieved. The resistance used was the one that gave maximal output potential in the latest set of results (2200 ohms) (Fig 4). It is known that, current = coulomb per time, while the area under the curve and above the baseline = (Coulomb / Time) x Time = Coulomb. From Table 5, the area under the curve and above the baseline was calculated to be 5.1 Coulombs using integration. Calculating Columbic Efficiency %: On addition of 1mL of 1M acetate Number of moles of acetate added = 1/1000 x 1 = 0.001 moles of acetate Number of moles of electrons available in 0.001 moles of acetate = 0.001 x 8 = 0.008 moles of eSince 1 moles of electrons = 96485 Coulombs, 0.008 moles of electrons = 96485 x 0.008 = 771.88 Coulombs. Hence, in theory, 0.001 moles of acetate should have 771.88 Coulombs. Columbic efficiency = ___________Coulombs calculated from the curve x 100__________ Theoretical amount of Coulombs in added amount of acetate 11 = 5.1 / 771.88 x 100 = 0.665% (which is an extremely low efficiency) At a resistance of 2200 ohms, the voltage stabilised at 61.5mV (0.028mA), showing that since the resistance is stable, an increase in voltage will cause an increase in current (I=V/R) (Fig 5). Once the 1mL of acetate was added, a slow increase in voltage occurred over about 7.2 hours, maxing out at 73mV (0.033mA). Once all the acetate was metabolised, the voltage decreased over 13.8 hours, going back to the baseline voltage of 61.5mV. The current baseline that was constructed over about 21 hours was used to find the area of the graph, which further assisted in calculating the coulombic efficiency. Figure 6: Correlation of acetate oxidation rate and current (Second batch results). The current shows the electron flow rate occurring in the MFC, which also equals the current in coulombs per second (C/s). As known, one mole of electrons (e-) equals 96485 C / mole e- (Faradays constant), therefore, to get the current in moles of e-/sec it would be (C/s) divided by 96485. To get the moles of acetate per hour it would be moles e- per second divided by 8 (acetate yields eight electrons (requires two moles of oxygen)) x3600 (assuming all electrons are used to make electricity). To get the acetate oxidation rate (moles of acetate/L/h), it would be moles of acetate per hour 12 divided by the volume of the anolyte (0.5L). This answer should equal to the current (linear correlation), however, it was off by a factor of about 111 in this experiment (Fig 6). The reason for this is due to the extremely low coulombic efficiency (0.665%) (decrease coulombic efficiency, decreases oxidation rate), as at low coulombic efficiencies, the chances of an accurate finding of the acetate oxidation rate decreases severely. The following day was when the MFC was when the culture was put in a continuous culture (chemostat). 50mL of acetate (1M) was pumped into the anode vessel everyday (vessel total volume = 500mL). Further on, the dilution rate is equal to the flow rate (L/h) divided by the volume (L). Therefore, the dilution rate equals (0.05/24)/0.5, which equals 0.0042 h-1, and the average treatment time, also known as the hydraulic retention time (HRT) equals 1/0.0042 h (240 h or 10 days). Figure 7: Chemostat polarisation curves. These results were taken five days after the chemostat was put into place (Fig 7; Table 3). As usual, an increase in current caused an eventual decrease in voltage. In the first chemostat polarization curve, there was quite a high increase in the voltage compared 13 to the batch culture (0.453V compared to 0.278V and 0.2724V) (Fig 7; Fig 1). Activation losses dominated at a voltage between 0.453V and 0.452V (may not have even occurred), while ohmic losses surged at a voltage between 0.452V and 0.412V. The linear drop in voltage (concentration loss) was evident when the voltage was between 0.412V and 0.0335V. Once again, if this polarisation curve was added to the other two, there would be a larger shift to the right and the graph would be starting at a higher point, showing that the voltage stayed higher at a higher current for a longer time, in comparison to the others. The second set of chemostat results were obtained the following day. A voltage of 0.556V occurred in the final set of results and this was the healthiest stage of the bacteria in this experiment (previous voltage of 0.453V) (Fig 7). Activation loss was not really evident but ohmic losses occurred at voltages between 0.557V and 0.535V, while concentration loss dominated at a voltage between 0.535V and 0.215V. Overall, it was clear to see that the highest voltages, over various currents, occurred in the last set of results that were conducted in this experiment. Figure 8: Chemostat power curves. 14 A new maximal output potential was achieved, being about 13 times higher than the previous maximum one (0.115mW compared to 0.0089mW) (Fig 8; Fig 4). This occurred at a current of 0.45mA and at a resistance of 560 ohms, which was almost 2000 less ohms than the final batch culture results. This, once again, showed that the bacteria were a lot stronger compared to the previous tests. The maximal volumetric power was 3.54x10-5 W/m3, which was also higher than the previous maximum result of 1.39x10-5 W/m3. The highest maximal output achieved in this experiment occurred at the final readings of the chemostat (0.48mW, compared to the previous maximum of 0.115mW) (Fig 8). The corresponding current was 1.5mA. The resistance decreased even more, settling at 220 ohms, emphasising that the bacteria just kept getting better. 0.002 W/m3 was the maximal volumetric power, also, being higher than the previous power (3.54x10-5 W/m3). Summing up, over time, it was clear to see that the bacteria improved their ability to make electricity. The maximum voltage increased over the four set of results (from 0.272V to 0.556V) and the voltages stayed higher over lower resistances. The resistance at which the power was maximal kept decreasing (2500 ohms to 220 ohms), also showing the improvement of bacteria over time (days/weeks). On top of this, a continuous improvement in the maximal volumetric power (1.39E-05 W/m3 to 0.0019 W/m3) and maximal power (0.003474 mW to 0.477164 mW) was evident, too. However, at a batch coulombic efficiency of only 0.665%, this shows that the bacteria, in the batch culture, were extremely weak. Discussion In this experiment, the behavioural characteristics of microbial fuel cell was investigated and observed. From the results, it confirmed the hypothesis that bacteria can be forced to act like a battery under certain conditions. The results evidently showed that the bacteria were getting better or more capable of charging up the 15 electric potential in the microbial chamber. This can be observed from Figure 3 and 7, showing a two fold increase in voltage readings from 0.272 V (first batch results) to 0.556 V (second chemostat results) at 1M ohms resistance. Other than voltage, the MFC also recorded better readings in key performance parameters, such as maximum power output at corresponding resistances. This is apparent from Figure 4 and 8 where the maximum power output increased from 0.0035 mW at 2500 ohms (first batch results) to 0.48 mW at 220 ohms (second chemostat results). The increase in maximum power output and voltage at decreasing resistance confirmed that the bacteria were improving in health; resulting in an increase in the amount of bacteria which can transfer electrons to the carbon anode. During the course of this experiment, the MFC ran in two feeding modes which were fed-batch and chemostat batch. However, the chemostat mode of this experiment is technically more of a semi-continuous batch mode. The reason for the phrase ‘semicontinuous’ was because the inflow and outflow of the bacteria anode chamber, though having the characteristics of a chemostat, was only semi-continuous as it had an inflow of 10 mL substrate 4 hours 48 minutes interval. A true chemostat has a continuous inflow and outflow. In the semi-continuous batch mode, the bacteria may be experiencing starvation during the 4 hours 48 minutes interval. This semicontinuous feeding schedule may affect the bacteria physiologically. The reason for the long interval is due to the limitations introduced by the pumping system which allows the slowest pumping rate of 10 mL per minute. The reason why 50 mL of 0.02 M of acetate was fed to the bacteria per day during the semi-continuous mode, was due to the fact that during the experiment for the coulombic efficiency in batch mode, the bacteria took more than 20 hours to fully metabolise 1 mL of 1 M acetate. The coulombic efficiency (CE) for this MFC in batch mode was 0.665 %. This is significantly lower than the usual CE published in literatures which range from 6080% [2]. From Figure 6, acetate oxidation rate was out by a factor of 111. This is because, the lower the CE, the more inaccurate will the acetate oxidation rate be. The 16 low CE may be due to the fact that 1 mL of 1 M acetate was used in getting the feed spike curve. This caused the long hours needed for the acetate added to be fully metabolised, which may result in inaccuracies. A aliquot of 30 uL of 1 M acetate should produce a more accurate result. The coulombic efficiency for the semicontinuous mode was not done as it was impossible to determine the amount of acetate present in the anode chamber at any one time without doing gas chromatography. However, the results also clearly showed that the MFC in semicontinuous batch mode was significantly more capable than the MFC in batch mode as its power output and maximum potential build up were both almost twice of that in batch mode. However, no further concrete conclusions can be made from this comparison as the biomass in both modes were not collected and measured. Further investigation is recommended in determining the amount of biomass present in semicontinuous batch and batch mode as it is expected that the amount of biomass in semicontinuous batch will be significantly more than those in batch mode. Further experiments should be conduced to find out whether the increase of potential was caused by the increase in amount of bacteria or simply the bacteria were becoming more efficient in donating electrons to the anode. Hence, it is recommended for further experiments, the weight of the carbon sponge to be weighed first before starting the experiment to allow the biomass to be measured. The effect of temperature on the electric potential produced by the bacteria is worth further investigations, as the increase in temperature to 30°C increases the electric potential significantly. Changing different substances at the cathode chamber may also produce different results worth investigating. 17 References Atkins, P. & De Paula, J. (2006) Atkins' Physical Chemistry, Oxford University Press. Gangrekar M.M & Shinde V.B (2006) Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresource Technology, 97, 543-550. Ieropoulos, I., Greenman, J., Melhuish, C. & Hart, J. (2005) Comparative study of three types of microbial fuel cell. Enzyme and Microbial technology, 37, 238-245. Larminie, J. & Dicks, A. (2003) Fuel Cell Systems Explained, West Sussex, John Wiley and Sons LTD. Liu, H., Ramanarayanan, R. & Logan, B. (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environmental Science and Technology, 38, 2281-2285. Min, B. & Logan, B. (2004) Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environmental Science and Technology, 38, 5809-5814. Mohan, S., Raghavulu, S., Srikanth, S. & Sarma, P. (2007) Bioelectricity production by mediatorless microbial fuel cell under acidophillic condition using wastewater as a substrate: Influence of substrate loading rate. Current Science, 92, 1720-1726. Moon, H., Chang, I. S. & Kim, B. H. (2006) Continuous electricity production from artificial wastewater using a mediator-less microbial fuel cell. Bioresource Technology, 97, 621-627. Serway, R. & Faughn, J. (2003) College Physics, Melbourne, Thompson Brooks\Cole. Stams, A., De Bok, F., Plugge, C., Van eekert, M., Dolfing, J. & Schraa, G. (2006) Exocellular electron transfer in anaerobic microbial communities. Environmental Microbiology, 8, 371-382. Williams, K. (Ed.) (1966) An Introduction to Fuel Cells, London, Elsevier Publishing. 18 Recommendations (Overview) Intrepid biotechnologists, congratulations on choosing the bioelectricity project. Here are a few of points to help you on your journey. 1 – The anode chamber is the brown sludgy one. The cathode chamber is the one that looks like cordial. Someone who marks your work will probably ask you that sometime, so try and remember it. If you can’t remember it, then leap forward keenly as if you are bursting with the answer, and Ralf will tell you to shutup and pick on someone else. 2 – The anode is not really anaerobic because the oxygen is bad for electricity production, it is because it stinks like real sewerage even though it is fake (at least they told me it was) 3 – The cathode solution is not very nice. I don’t think there is even any real cordial in there. Maybe the cyanide bit is a hint. 4 – Ralf likes pumps, so put one in there early on, even if it is just for looks. That way you will still get to do nothing for the first two weeks, but no-one will bug you about progress. It doesn’t hurt to keep him happy, and not many people are so easily amused. 5 – Don’t worry so much about polarisation curves. I think it will give someone a headache to mark anyway. Just put a pump on it and play around with lots of different things, I would suggest dilution rate and different oxidising agents in the cathode chamber. Measure the current which you achieve at steady state. It is quicker too. If anyone actually figures out what a polarisation curve is, can you please email me? 6 – Kayu is the most helpful, knowledgeable and genuinely kind demonstrator you will ever come across. Pay attention to this guru. Even though he is quiet he is packing the most powerful MFC in the world today in that lab of his. He will probably rule the world one day by solving the energy crisis with a car that runs on sewerage with the added bonus of not having to stop for toilet breaks on long trips. 19 Recommendations (In detail) 1. It is best to place the MFC into a tank of 30°C as early as possible. This is the optimum temperature for the bacteria to live in. 2. Ensure the water level in the incubation tank is at the correct height level at all times. 3. The setup should be done in such a way so that the wire connecting to the two electrodes are not being disturbed too much. ie. Use crocodile clip wires in between the electrodes and the resistor & voltmeter. 4. Current should be calculated using Ohm’s law instead of reading off the voltmeter. 5. The MFC equipments are all very expensive. Handle with care. 6. It is recommended to go straight into chemostat mode and conduct experiments in that mode instead of using precious time doing a batch mode. 7. A pump which can pump very small amounts of substrate for very short intervals of time should be secured as early as possible for the experiment. 8. Stirring rate is recommended to be at around 200 rpm. Too high a stirring rate may dislodge the electricity producing bacteria from the anode. 9. The total concentration of acetate in the anode chamber at any one time is recommended to be not more than 0.1 mM to 0.5 mM. 10. After running the MFC for 3 to 5 days, the blackish sludge can be poured away carefully and slowly and replace the anode chamber with synthetic wastewater. Ensure that the carbon sponge anode is not disturbed too much during the transition as the electric producing bacteria should be adhering themselves to it. Initial voltage may be lowered drastically but will increase dramatically soon after. 11. pH should be monitored frequently to ensure that the pH does not rise or fall too much. 20 12. If carbon source was added into the synthetic wastewater, proper measures should be taken to prevent or delay its contamination with bacteria in the surroundings. 13. Ensure that the circuit is not opened for too long as the bacteria may die off due to lack of means to transfer electrons. 14. If any stirring is to be introduced in any thing, ensure that it is as air tight as possible to reduce oxygen dissolving into the system. The system is to be keep as anaerobic as possible for maximum electricity production. 15. Ferricyanide should be changed in the cathode when it turns turbid or loses colour. Alternatively, peroxide can be added to reoxidise it. 16. If a pump is added, a compromise in the air-tightness of the system may occur. This is because an opening need to be introduce so that an inflow and outflow can occur. Without the opening, the pressure in the vacuum will prevent the pump from doing its job. Measures can be taken to both reduce the oxygen dissolution as well as contamination. 17. Prior knowledge on MFC and electrochemistry is preferable to do this experiment. The first week should be used to familiarise these concepts from literature. 18. Using a polarisation and power curve is the best way to illustrate that the bacteria culture in the anode chamber is getting enriched as the experiment proceeds. Similarly, columbic efficiency should improve over time. 19. When producing the polarisation/power curves, there is no need to wait for the voltage to completely stabilise. It could take half a day to a day to stabilise. As soon as the increase in voltage, during the charging up, is significantly slow (about 0.1 mV every 10 to 15 minutes) it is good enough to be considered as stabilised. Proceed with the experiment from there. 20. Then doing the columbic efficiency curve, 30 uL of 1 M acetate solution is more than enough to produce the “feed spike”. Adding more than that will result in long hours of waiting time for all the added acetate to be fully metabolised. 21 21. As this experiment is very new and you will probably be the second group to be doing this, MAKE SURE you know exactly what your experiment is going to achieve and how are you going to carry it out latest by 3rd week. This is probably loads of things you can find out about MFCs but be realistic and choose those that interest you most and discuss with your supervisors. 22. You will probably hear a lot of recommendations on what you can do with your experiment. Listen to those BUT choose only those that you think you are able to carry out. Time, equipment, complexity of the tasks should be some of your considering factors. 23. During the first week, have a serious meeting and layout house rules. Identify those members who are most likely not going to have much contribution and lay down the terms and conditions. This is essential and should be done as soon as possible. In summary, this project is relatively easy, and will probably be a lot easier for you than for us. It will better if any group doing this project has a bit of Chemistry and Physics background, don’t panic, just the basic stuff. You can get very technical on this one, but I would suggest playing around with more things and collecting simpler information, maybe columbic efficiency and current output at steady state. I think we made a valuable contribution to science too. We probably generated enough power to run a single LED to read by for 2 seconds. It is amazing what you can read in two seconds. Other methods of power generation from sewerage might also be worth investigating. Like putting it on your garden, growing potatoes, feeding them to rats, and making the rats generate power on a treadmill. Try and put a pump in there though to keep Ralf happy (BLESS HIM). Good luck! 22 Acknowledgement We would like to thank our lecturer, Dr Ralf, for his valuable guidance and advices for our project. We would like to thank our Mentor, Kayu Cheng who assisted us in configuring and the setting up of Microbial Fuel Cell. 23 Appendix Materials and equipment 1L Sodium acetate 1.0M for batch culture 1L Sodium acetate 0.02M for chemostat 1L activated sludge 2 electronic timers (LT48W) 2 magnetic stirrer bars 2000mL synthetic waste added with 2.mL of trace elements 3 Terumu needles (0.65 x 32mm) with syringes 70% ethanol spray Aquarium (500mL) Bandages (for insulation purpose) Cotton wool (for insulation purpose) Crocodile clips wire Distilled water Electronic speed adjustable pump (Chemap AG) Esky box Hot plate stirrer MaCartney bottles Oxygen probe (Hanna instrument HI9145) Pen knife pH meter Pipette tips 24 Pipette (P200) Potassium Ferricyanide ~ 50mM at pH7 + 100mL Phosphate biffer ~ 100mM PVC fasteners Trace element (1.25mL/L) Voltmeter Rubber pipes (approximately 1.5 meters for whole experiment) Resistor Scissor 1L Synthetic wastewater consists 20mL of sodium acetate 1.0M, 1.25mL trace elements, 480mg NaHCO3, 95.5mg NH4Cl, 10.5mg K2HPO4, 5.25mg KH2PO4, 63.1mg CaCl2.2H20 and 19.2mg MgSO4.7H2O. 25 A set of MFC which consists of the following: Figure 9: Batch culture set up 26 Figure 10: Chemostat set up 27 Figure 11: In detail of chemostat set up 28 Table 1: First set of results taken to form a power and polarisation curve. Resistance Voltage Voltage (Ohms) (V) (mV) Current (mA) Power (mW) 1000000 560000 330000 220000 100000 68000 47000 33000 4700 2500 1150 1000 660 550 330 150 56 33 5 0.000272 0.000486 0.000826 0.001238 0.00272 0.003976 0.005719 0.008073 0.025638 0.03728 0.053739 0.055 0.058485 0.06 0.066061 0.068667 0.096429 0.09697 0.098 7.4E-05 0.000133 0.000225 0.000337 0.00074 0.001075 0.001537 0.002151 0.003089 0.003474 0.003321 0.003025 0.002258 0.00198 0.00144 0.000707 0.000521 0.00031 4.8E-05 0.272 0.2724 0.2725 0.2724 0.272 0.2704 0.2688 0.2664 0.1205 0.0932 0.0618 0.055 0.0386 0.033 0.0218 0.0103 0.0054 0.0032 0.00049 272 272.4 272.5 272.4 272 270.4 268.8 266.4 120.5 93.2 61.8 55 38.6 33 21.8 10.3 5.4 3.2 0.49 Volumetric Power (W/m3) 2.96E-07 5.3E-07 9E-07 1.35E-06 2.96E-06 4.3E-06 6.15E-06 8.6E-06 1.24E-05 1.39E-05 1.33E-05 1.21E-05 9.03E-06 7.92E-06 5.76E-06 2.83E-06 2.08E-06 1.24E-06 1.92E-07 Table 2: Second set of results taken to form a power and polarisation curve Resistance Voltage Voltage Current (Ohms) (V) (mV) (mA) Power (mW) 1000000 560000 330000 220000 100000 68000 47000 33000 22000 15000 10000 6800 4700 2200 1500 7.73E-05 0.000137 0.000233 0.000349 0.000762 0.001104 0.001574 0.002214 0.003108 0.004315 0.005944 0.007564 0.008725 0.008858 0.00769 0.278 0.277 0.277 0.277 0.276 0.274 0.272 0.2703 0.2615 0.2544 0.2438 0.2268 0.2025 0.1396 0.1074 278 277 277 277 276 274 272 270.3 261.5 254.4 243.8 226.8 202.5 139.6 107.4 0.000278 0.000495 0.000839 0.001259 0.00276 0.004029 0.005787 0.008191 0.011886 0.01696 0.02438 0.033353 0.043085 0.063455 0.0716 29 Volumetric Power (W/m3) 3.09E-07 5.48E-07 9.3E-07 1.4E-06 3.05E-06 4.42E-06 6.3E-06 8.86E-06 1.24E-05 1.73E-05 2.38E-05 3.03E-05 3.49E-05 3.54E-05 3.08E-05 1000 820 330 150 56 33 10 5 0.0796 0.0682 0.0308 0.0145 0.0055 0.0034 0.0011 0.00006 79.6 68.2 30.8 14.5 5.5 3.4 1.1 0.6 0.0796 0.083171 0.093333 0.096667 0.098214 0.10303 0.11 0.12 0.006336 0.005672 0.002875 0.001402 0.00055 0.00035 0.000121 0.000072 2.53E-05 2.27E-05 1.15E-05 5.61E-06 2.16E-06 1.4E-06 4.84E-07 2.88E-07 Table 3: First set of chemostat results taken to form a power and polarisation curve Resistance Voltage Voltage Current (Ohms) (V) (mV) (mA) Power (mW) 1000000 560000 330000 150000 82000 47000 10000 4700 1000 560 220 100 47 22 5 0.000205 0.000366 0.000622 0.001368 0.002492 0.004366 0.019097 0.036116 0.094864 0.115207 0.084073 0.040196 0.023878 0.011636 0.002738 0.453 0.453 0.453 0.453 0.452 0.453 0.437 0.412 0.308 0.254 0.136 0.0634 0.0335 0.016 0.0037 453 453 453 453 452 453 437 412 308 254 136 63.4 33.5 16 3.7 0.000453 0.000809 0.001373 0.00302 0.005512 0.009638 0.0437 0.08766 0.308 0.453571 0.618182 0.634 0.712766 0.727273 0.74 Volumetric Power (W/m3) 8.21E-07 1.47E-06 2.49E-06 5.47E-06 9.97E-06 1.75E-05 7.64E-05 0.000144 0.000379 0.000461 0.000336 0.000161 9.55E-05 4.65E-05 1.1E-05 Table 4: Second set of chemostat results taken to form a power and polarisation curve Resistance Voltage Voltage Current (Ohms) (V) (mV) (mA) Power (mW) 1000000 560000 330000 150000 82000 47000 10000 0.000309 0.000552 0.000937 0.002061 0.003784 0.006601 0.03003 0.556 0.556 0.556 0.556 0.557 0.557 0.548 556 556 556 556 557 557 548 0.000556 0.000993 0.001685 0.003707 0.006793 0.011851 0.0548 30 Volumetric Power (W/m3) 1.23654E-06 2.20811E-06 3.7471E-06 8.24363E-06 1.51341E-05 2.64042E-05 0.000120122 4700 1000 560 220 100 47 22 5 0.535 0.473 0.428 0.324 0.215 0.105 0.052 0.012 535 473 428 324 215 105 52 12 0.11383 0.473 0.764286 1.472727 2.15 2.234043 2.363636 2.4 0.060899 0.223729 0.327114 0.477164 0.46225 0.234574 0.122909 0.0288 31 0.000243596 0.000894916 0.001308457 0.001908655 0.001849 0.000938298 0.000491636 0.0001152 Table 5: Calculations for Columbic Efficiency. Time sec Normalized Curr Mean Curr with baseline curr in intrv. Coulomb mC/s mC C interv. voltage Resistance Current sec mV Ohm mA mC/s mC/s 61.5 999 0.06156 0.06156 0.0000 0 3600 3600 61.5 1000 0.0615 0.0615 -0.0001 0.062 222 0.222 4500 900 61.8 1000 0.0618 0.0618 0.0002 0.062 55 0.055 4920 420 62.1 1000 0.0621 0.0621 0.0005 0.062 26 0.026 5520 600 62.3 1000 0.0623 0.0623 0.0007 0.062 37 0.037 6120 600 62.6 1000 0.0626 0.0626 0.0010 0.062 37 0.037 6720 600 62.8 1000 0.0628 0.0628 0.0012 0.063 38 0.038 7320 600 63 1000 0.063 0.063 0.0014 0.063 38 0.038 7920 600 63.2 1000 0.0632 0.0632 0.0016 0.063 38 0.038 8520 600 63.4 1000 0.0634 0.0634 0.0018 0.063 38 0.038 9120 600 63.5 1000 0.0635 0.0635 0.0019 0.063 38 0.038 9720 600 63.6 1000 0.0636 0.0636 0.0020 0.064 38 0.038 12720 3000 65.3 1000 0.0653 0.0653 0.0037 0.064 193 0.193 32 13320 600 65.7 1000 0.0657 0.0657 0.0041 0.066 39 0.039 16320 3000 68.1 1000 0.0681 0.0681 0.0065 0.067 201 0.201 16920 600 68.4 1000 0.0684 0.0684 0.0068 0.068 41 0.041 17520 600 68.9 1000 0.0689 0.0689 0.0073 0.069 41 0.041 18120 600 69.4 1000 0.0694 0.0694 0.0078 0.069 41 0.041 18720 600 69.7 1000 0.0697 0.0697 0.0081 0.070 42 0.042 20520 1800 71 1000 0.071 0.071 0.0094 0.070 127 0.127 24120 3600 72.6 1000 0.0726 0.0726 0.0110 0.072 258 0.258 24720 600 72.65 1000 0.07265 0.07265 0.0111 0.073 44 0.044 25920 1200 73 1000 0.073 0.073 0.0114 0.073 87 0.087 26520 600 73 1000 0.073 0.073 0.0114 0.073 44 0.044 27120 600 72.9 1000 0.0729 0.0729 0.0113 0.073 44 0.044 27720 600 72.8 1000 0.0728 0.0728 0.0112 0.073 44 0.044 28320 600 72.7 1000 0.0727 0.0727 0.0111 0.073 44 0.044 28920 600 72.6 1000 0.0726 0.0726 0.0110 0.073 44 0.044 29520 600 72.5 1000 0.0725 0.0725 0.0109 0.073 44 0.044 30120 600 72.4 1000 0.0724 0.0724 0.0108 0.072 43 0.043 33 30720 600 72.3 1000 0.0723 0.0723 0.0107 0.072 43 0.043 31320 600 72.2 1000 0.0722 0.0722 0.0106 0.072 43 0.043 31920 600 72.1 1000 0.0721 0.0721 0.0105 0.072 43 0.043 32520 600 72 1000 0.072 0.072 0.0104 0.072 43 0.043 33120 600 71.8 1000 0.0718 0.0718 0.0102 0.072 43 0.043 33720 600 71.7 1000 0.0717 0.0717 0.0101 0.072 43 0.043 34320 600 71.5 1000 0.0715 0.0715 0.0099 0.072 43 0.043 34920 600 71.4 1000 0.0714 0.0714 0.0098 0.071 43 0.043 35520 600 71.2 1000 0.0712 0.0712 0.0096 0.071 43 0.043 36120 600 70.3 1000 0.0703 0.0703 0.0087 0.071 42 0.042 36720 600 70 1000 0.07 0.07 0.0084 0.070 42 0.042 37320 600 69.6 1000 0.0696 0.0696 0.0080 0.070 42 0.042 60120 22800 65 1000 0.065 0.065 0.0034 0.067 1534 1.534 72120 12000 62.1 1000 0.0621 0.0621 0.0005 0.064 763 0.763 72720 600 62 1000 0.062 0.062 0.0004 0.062 37 0.037 73320 600 61.8 1000 0.0618 0.0618 0.0002 0.062 37 0.037 73920 600 61.7 1000 0.0617 0.0617 0.0001 0.062 37 0.037 34 74520 600 61.5 1000 0.0615 0.0615 -0.0001 0.062 37 0.037 75120 600 61.4 1000 0.0614 0.0614 -0.0002 0.061 37 0.037 75720 600 61.3 1000 0.0613 0.0613 -0.0003 0.061 37 0.037 76320 600 61.3 1000 0.0613 0.0613 -0.0003 0.061 37 0.037 76920 600 61.3 1000 0.0613 0.0613 -0.0003 0.061 37 0.037 Sum of C 5.1 35 36