* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download GRADE 12A: Chemistry 6
Survey
Document related concepts
Transcript
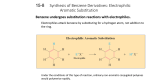
GRADE 12A: Chemistry 6 UNIT 12AC.6 11 hours Aromatic organic chemistry About this unit Previous learning Resources This unit is the sixth of eight units on chemistry for Grade 12 advanced. To meet the expectations of this unit, students should already know how to distinguish between and bonds and recognise the relative unreactivity of the arene ring. The main resources needed for this unit are: The unit is designed to guide your planning and teaching of chemistry lessons. It provides a link between the standards for science and your lesson plans. The teaching and learning activities should help you to plan the content and pace of lessons. Adapt the ideas to meet your students’ needs. For consolidation activities, look at the scheme of work for Grade 11A. You can also supplement the activities with appropriate tasks and exercises from your school’s textbooks and other resources. • 0.01 mol dm–3 potassium manganate(VII), 1 mol dm–3 sulfuric acid, methyl benzene • class set of student whiteboards Expectations By the end of the unit, students know the fundamental chemistry of arenes and substituted arenes and describe the production of the more important derivatives of benzene. They explain the stability of the benzene ring in terms of electron delocalisation. Students who progress further understand the mechanism of electrophilic substitution reactions and are able to predict the effect of substitutions on the aromatic ring. Introduce the unit to students by summarising what they will learn and how this builds on earlier work. Review the unit at the end, drawing out the main learning points, links to other work and real world applications. 489 | Qatar science scheme of work | Grade 12 advanced | Unit 12AC.6 | Chemistry 6 • molecular model kits • methyl benzoate, concentrated sulfuric acid, concentrated nitric acid, ice • methylphenol, phenol, phenylamine, sodium nitrate(III), sodium hydroxide, napthalen-2-ol, ice, fume cupboard • Internet access Key vocabulary and technical terms Students should understand, use and spell correctly: • arenes, aromatic • electrophilic substitution • azo dyes, coupling, diazotisation © Education Institute 2005 Standards for the unit 11 hours 4 hours Nomenclature of arenes 1 hour Bonding in arenes 4 hours Reactions of arenes 2 hours Azo dyes Unit 12AC.6 SUPPORTING STANDARDS 11A.24.1 Know, interpret and use the CORE STANDARDS Grade 12 standards 112A.23.1 Interpret and use the nomenclature and structural formulae of the following nomenclature and molecular and structural formulae of the following classes of compound: • alkanes and alkenes; • halogenoalkanes; • alcohols; • aldhehydes and ketones; • carboxylic acids, esters and acyl chlorides; • amines, nitriles, amides and amino acids 11A.18.10 Describe covalent bonding in terms of orbital overlap, giving (sigma) and (pi) bonds; explain bond shape and angles in ethane, ethene and benzene in terms of and bonds. 11A.24.18 Describe the chemistry of arenes (such as benzene and methylbenzene) and show an understanding of the relative unreactivity of the aromatic ring compared with an isolated double bond; know that the chemistry of side chains is similar to that of aliphatic compounds. 11A.24.20 Compare the preparation and properties of bromobenzene with bromoethane to show the effect of the benzene ring. 11A.24.19 Know the chemistry of phenol, as exemplified by its reactions with bases and sodium, and know of its common use as a mild disinfectant. 11A.24.20 Show an understanding of the broad issues relating to social benefits and environmental costs associated with the organic chemical industry. EXTENSION STANDARDS classes of compound: • arenes; • halogenoarenes; • phenols; • aromatic aldehydes and ketones; • aromatic carboxylic acids, esters and acyl chlorides; • aromatic amines, nitriles, amides and amino acids. 12A.23.2 Describe the shapes of the ethane, ethene and benzene molecules in terms of and carbon–carbon bonds. 12A.23.3 Describe the chemistry of arenes (such as benzene and methylbenzene), as exemplified by substitution reactions with electrophiles, nitration and oxidation of the side chain. 12A.23.4 Understand the mechanism of electrophilic substitution in arenes and the effect of the delocalisation of electrons in arenes in such reactions. 12A.23.5 Know the chemistry of phenol, as exemplified by its reactions with bases and sodium and by electrophilic substitution in the aromatic ring. 12A.23.6 Describe the formation of aromatic amines by the reduction of nitroarenes. 12A.23.7 Describe the production of azo-dyes from phenylamine and understand their commercial importance. 490 | Qatar science scheme of work | Grade 12 advanced | Unit 12AC.6 | Chemistry 6 © Education Institute 2005 Activities Unit 12AC.6 Objectives Possible teaching activities 4 hours For each of the classifications of compound listed in the standard (i.e. arenes; halogenoarenes; phenols; aromatic aldehydes and ketones; aromatic carboxylic acids, esters and acyl chlorides; aromatic amines, nitriles, amides and amino acids) go through the following method to consolidate student understanding of nomenclature: Nomenclature of arenes Interpret and use the nomenclature and structural formulae of the following classes of compound: • arenes; • halogenoarenes; • phenols; School resources Use this column to note your own school’s resources, e.g. textbooks, worksheets. • Write the name of the relevant class of aliphatic compound previously met in the course (e.g. halogenoalkanes if you intend teaching halogenoarenes) and a structure of examples of aliphatic analogues (if appropriate) on the board or OHP. • Ask students to draw the structure of the named compound and the name of the structure onto a small whiteboard and hold it up for you to see. • aromatic carboxylic acids, esters and acyl chlorides; • The degree of revision of nomenclature for these compounds will depend on students’ responses. If they appear to be confident, just summarise the rules for nomenclature for this class of compound. If they are not confident, reinforce the rules and give them more examples of compound names and structures. Let them work in pairs and mark each other’s work, explaining any incorrect answers to their partner. • aromatic amines, nitriles, amides and amino acids. • Explain to the whole class the extra information needed to be able to name the aromatic analogues. • aromatic aldehydes and ketones; Notes Repeat the above process for the aromatic analogues of the aliphatic compounds you have covered. 1 hour Bonding in arenes Describe the shapes of the ethane, ethene and benzene molecules in terms of and carbon–carbon bonds. Revise, through a question and answer session, the shape and type of bonding in ethane and ethene molecules. Give students data relating to the enthalpy change of hydrogenation of cyclohexene and ask them to draw an enthalpy level diagram for the hydrogenation of ‘cyclohexatriene’. Provide them with data for the enthalpy change of hydrogenation of benzene and ask them to superimpose this value onto their enthalpy level diagram. Provide them with data for C–C and CC as well as the carbon–carbon bond length in benzene. Ask them to explain this. Ask students to use the library or the Internet to research the work of Friedrich Kekulé and, working in small groups, produce a poster of his work. 491 | Qatar science scheme of work | Grade 12 advanced | Unit 12AC.6 | Chemistry 6 Two good websites are: • www.chemguide.co.uk/basicorg/bonding/ benzene1.html • classes.yale.edu/chem220a/studyaids/ history/chemists/kekule.html ICT opportunity: Use of the Internet. Enquiry skills 12A.2.1, 12A.2.4 © Education Institute 2005 Objectives 4 hours Reactions of arenes Describe the chemistry of arenes (such as benzene and methylbenzene), as exemplified by substitution reactions with electrophiles, nitration and oxidation of the side chain. Understand the mechanism of electrophilic substitution in arenes and the effect of the delocalisation of electrons in arenes in such reactions. Know the chemistry of phenol, as exemplified by its reactions with bases and sodium and by electrophilic substitution in the aromatic ring. Possible teaching activities Notes –3 –3 Tell students to mix equal volumes of 0.01 mol dm potassium manganate(VII) and 1 mol dm sulfuric acid with a few drops of methyl benzene. Tell them to observe colour changes and so determine whether the manganate(VII) oxidised the methylbenzene. School resources Safety: Methyl benzene is flammable. Draw the class together and give them the equation for this and other similar reactions. Explain to the whole class a general mechanism for the electrophilic substitution of benzene. Ask students to use the library or the Internet to research the conditions needed for bromination, chlorination, nitration, sulfonation, Friedel-Crafts alkylation and Friedel-Crafts acylation. Ask them, working individually, to produce a chart identifying the reaction conditions, the mechanism and the electrophile for each of these reactions. ICT opportunity: Use of the Internet. Give students the dipole moments for a number of monosubstituted benzene rings (e.g. phenylamine, chlorobenzene) and ask them to classify these into two groups: those that are electron-withdrawing from the aromatic ring and those that are electron-releasing. Ask them to predict the effect of each group on reactivity in electrophilic substitution reactions. + Guide students through drawing canonical forms for the attack of NO at the ortho position of + phenol then ask them to draw similar diagrams to show the canonical forms when NO attacks at the meta position of phenol. Through class discussion and question and answer, explain why the former is preferential. + Repeat the process for the attack of NO2 on benzoic acid. Ask students, in pairs, to carry out an electrophilic substitution reaction. A suitable one is the nitration of chilled methyl benzoate in concentrated sulfuric acid, with a chilled nitrating mixture of concentrated sulfuric acid and concentrated nitric acid, keeping the temperature below 10 °C. Crystallise the product out over ice. Safety: Methyl benzoate is harmful, concentrated sulfuric acid and concentrated nitric acid are corrosive. Enquiry skill 12A.4.1 Revise the reactions of phenol with bases and sodium (done as a practical in Grade 11) with a quiz. 2 hours Azo dyes Describe the formation of aromatic amines by the reduction of nitroarenes. Describe the production of azo-dyes from phenylamine and understand their commercial importance. Ask students to use the library or the Internet to research the work of Otto Witt and describe his contribution to the azo dye industry. Also ask them to research azo dye production worldwide and present the data in a suitable format. Describe to the whole class the stages of synthesising an azo dye (i.e. diazotisation and coupling). Give students structures of a number of pairs of reactants and ask them (individually) to draw out the structure of the resultant dye. Also carry out the process in reverse. Let students produce a range of azo dyes using phenylamine and ethyl-4-aminobenzoate as amines to prepare as diazonium salts. Tell them to prepare each diazonium salt by adding the aryl amine to cooled dilute hydrochloric acid, cool below 5 °C. They should then add a cooled solution of sodium nitrate(III), not allowing the temperature to rise above 5 °C. To prepare the coupling agents, chill phenol, 3-methylphenol and napthalen-2-ol, each made up in alkaline solution, to below 5 °C. Tell students, working in pairs, to add each coupling agent slowly to each diazonium salt in turn and observe the colours of the resultant dyes. 492 | Qatar science scheme of work | Grade 12 advanced | Unit 12AC.6 | Chemistry 6 ICT opportunity: Use of the Internet. Video footage of coupling reactions can be seen on: www.uni-regensburg.de/Fakultaeten/ nat_Fak_IV/Organische_Chemie/Didaktik/ Keusch/D-azo-e.htm. Safety: Methylphenol, phenol, phenylamine and sodium nitrate(III) are toxic. Methylphenol, phenol and sodium hydroxide solution are corrosive. Sodium nitrate(III) is an oxidising agent. Napthalen-2-ol is harmful. Gloves and goggles must be worn at all times. The work must be done in a fume cupboard. Enquiry skills 12A.2.2, 12A.2.3, 12A.4.1 © Education Institute 2005 Assessment Examples of assessment tasks and questions Assessment Set up activities that allow students to demonstrate what they have learned in this unit. The activities can be provided informally or formally during and at the end of the unit, or for homework. They can be selected from the teaching activities or can be new experiences. Choose tasks and questions from the examples to incorporate in the activities. Unit 12AC.6 Notes School resources If bromine water is added to a solution of phenylamine, a white precipitate of 2,4,6tribromophenylamine forms. Explain why the reaction occurs so readily. Suggest why the benzenediazonium ion attacks the para rather than the ortho position on phenol. Draw the structure of the azo compound produced in this reaction. Give a generic mechanism for the electrophilic substitution of benzene with an electrophile E+. Why is this reaction classified as an electrophilic substitution? Research and present a paper on the role of azo dyes in the organic chemical industry. Focus on the reasons for and nature of the development of the industry. Reference clearly any sources of information. 493 | Qatar science scheme of work | Grade 12 advanced | Unit 12AC.6 | Chemistry 6 © Education Institute 2005 494 | Qatar science scheme of work | Grade 12 advanced | Unit 12AC.6 | Chemistry 6 © Education Institute 2005