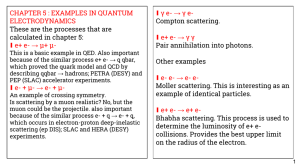
CHAPTER 5 : EXAMPLES IN QUANTUM γ e- → γ e- ∎ ELECTRODYNAMICS
... R = σ ( e e → hadrons ) / σ ( e ebar → μ μbar) . The underlying process in hadron production is e- + e+ → q + qbar. ...
... R = σ ( e e → hadrons ) / σ ( e ebar → μ μbar) . The underlying process in hadron production is e- + e+ → q + qbar. ...
Quantum Strategies V 82, N 5
... quantum cryptography, and quantum communication. In each case, representation in a quantum system provides advantages over the classical situation: Simon’s quantum algorithm to identify the period of a function chosen by an oracle is more efficient than any deterministic or probabilistic algorithm [ ...
... quantum cryptography, and quantum communication. In each case, representation in a quantum system provides advantages over the classical situation: Simon’s quantum algorithm to identify the period of a function chosen by an oracle is more efficient than any deterministic or probabilistic algorithm [ ...
PROJECTIVE AND CONFORMAL STRUCTURES IN GENERAL
... “..an element of wholeness, so to speak, in the physical processes, a feature going far beyond the old doctrine of the restricted divisibility of matter. This element is called the universal quantum of action. It was discovered by Max Planck in the first year of this century and came to inaugurate a ...
... “..an element of wholeness, so to speak, in the physical processes, a feature going far beyond the old doctrine of the restricted divisibility of matter. This element is called the universal quantum of action. It was discovered by Max Planck in the first year of this century and came to inaugurate a ...
Powerpoint format
... 1. The system is put into a superposition of all possible states, each weighted by its probability amplitude (= a complex number ci) E.g. Qubits for 2 electrons = c1 |00> + c2 |01> + c3 |10> + c4 |11> 2. The system evolves according to quantum principles: 1. Unitary matrix operation: describes how s ...
... 1. The system is put into a superposition of all possible states, each weighted by its probability amplitude (= a complex number ci) E.g. Qubits for 2 electrons = c1 |00> + c2 |01> + c3 |10> + c4 |11> 2. The system evolves according to quantum principles: 1. Unitary matrix operation: describes how s ...
Multiphoton antiresonance M. I. Dykman and M. V. Fistul
... periods 2 / 共g兲 are the same.10 When the motion is quantized, 共g兲 gives the distance between the energy levels. Therefore if, for some ␦ and , two levels that correspond to the external and internal trajectories coincide with each other, many levels will coincide pairwise as well. Level splitti ...
... periods 2 / 共g兲 are the same.10 When the motion is quantized, 共g兲 gives the distance between the energy levels. Therefore if, for some ␦ and , two levels that correspond to the external and internal trajectories coincide with each other, many levels will coincide pairwise as well. Level splitti ...
Thursday afternoon
... Recapitulation of Class 4 Hartree-Fock approximation gives an answer that is worse than if we just ignored interactions! ...
... Recapitulation of Class 4 Hartree-Fock approximation gives an answer that is worse than if we just ignored interactions! ...
Document
... Integratable with direct solid state photon sources (no need to up/down convert) Large existing infrastructure for nanofabrication High temperature operation – Compared to a dilution refrigerator CHALLENGE: spatial placement and ...
... Integratable with direct solid state photon sources (no need to up/down convert) Large existing infrastructure for nanofabrication High temperature operation – Compared to a dilution refrigerator CHALLENGE: spatial placement and ...
Problem set 4 Engel P7
... makes use of other things we already know. The average value < p x2 > is related to the average value of the kinetic energy, T: < p x2 >/2m=
Also, by conservation of energy
+ = E
where is the average value of the potential energy. Re-writing, we have
< p x2 > = 2m [E - ]
Here’s the ...
... makes use of other things we already know. The average value < p x2 > is related to the average value of the kinetic energy, T: < p x2 >/2m=
The Integer Quantum Hall Effect
... The Hall e↵ect has played already an interesting role in classical electrodynamics, since it provides a unique tool for determining the sign of the current carriers. This task is challenging since the electric current produced by particles of charge q moving at velocity v, is the same as that produc ...
... The Hall e↵ect has played already an interesting role in classical electrodynamics, since it provides a unique tool for determining the sign of the current carriers. This task is challenging since the electric current produced by particles of charge q moving at velocity v, is the same as that produc ...
Chapter 3 Basic quantum statistical mechanics of spin
... As with two sites, the ferromagnetic ground state is a multiplet, whereas the antiferromagnetic is a singlet. For a general N -site ferromagnet, the completely ferromagnetic states (all spins up or all spins down) are exact ground states of H. This suggests using an order parameter for ferromagnetis ...
... As with two sites, the ferromagnetic ground state is a multiplet, whereas the antiferromagnetic is a singlet. For a general N -site ferromagnet, the completely ferromagnetic states (all spins up or all spins down) are exact ground states of H. This suggests using an order parameter for ferromagnetis ...
Chapter 28: Quantum Physics
... Typically the excited states of electrons have lifetimes of about 10-8 seconds. To make a laser, the material must have metastable states with lifetimes of about 10-3 seconds. This allows for a population inversion in which more electrons are in a higher energy state rather than in a lower energy ...
... Typically the excited states of electrons have lifetimes of about 10-8 seconds. To make a laser, the material must have metastable states with lifetimes of about 10-3 seconds. This allows for a population inversion in which more electrons are in a higher energy state rather than in a lower energy ...
A commentary on Eric Scerri`s paper “Has Quantum Mechanics
... their chemistry was used by Niels Bohr to “deduce” the periodic table (see, e.g., Pais, 1991). In what follows, we’ll concentrate on the electronic properties of atoms as revealed by their spectra. In quantum mechanics, only few problems can be solved exactly (i.e., analytically). These include the ...
... their chemistry was used by Niels Bohr to “deduce” the periodic table (see, e.g., Pais, 1991). In what follows, we’ll concentrate on the electronic properties of atoms as revealed by their spectra. In quantum mechanics, only few problems can be solved exactly (i.e., analytically). These include the ...
Conclusive Exclusion of Quantum States
... well known result [1] that this can be done with certainty if and only if all of the states in the set of preparations are orthogonal to one another. By allowing inconclusive measurement outcomes [2–4] or accepting some error probability [5], strategies can be devised to tackle the problem of discri ...
... well known result [1] that this can be done with certainty if and only if all of the states in the set of preparations are orthogonal to one another. By allowing inconclusive measurement outcomes [2–4] or accepting some error probability [5], strategies can be devised to tackle the problem of discri ...
Quantum Hall effect in three-dimensional layered systems Yigal Meir
... out the separate transitions even for a finite number of layers. It is known that there may occur transitions between the expected adiabatic behavior to a different behavior ~as a function of, e.g., the tunneling matrix element!, even for the two-layer problem,21 and it remains to be seen if such a ...
... out the separate transitions even for a finite number of layers. It is known that there may occur transitions between the expected adiabatic behavior to a different behavior ~as a function of, e.g., the tunneling matrix element!, even for the two-layer problem,21 and it remains to be seen if such a ...
Document
... A hydrogen atom electron is excited to an energy of −13.6/4 eV. How many different quantum states could the electron be in? That is, how many wave functions ynℓm have this energy? ...
... A hydrogen atom electron is excited to an energy of −13.6/4 eV. How many different quantum states could the electron be in? That is, how many wave functions ynℓm have this energy? ...
the problem book
... breaks (i.e., at small lengths), the free energy, F = E − T S, of the spring scales similarly but not exactly like an ordinary spring’s potential energy; it satisfies the relation F/M = 12 kx2 , where M is the mass of the spring, x is its length per unit mass, k is related (but not identical) to the ...
... breaks (i.e., at small lengths), the free energy, F = E − T S, of the spring scales similarly but not exactly like an ordinary spring’s potential energy; it satisfies the relation F/M = 12 kx2 , where M is the mass of the spring, x is its length per unit mass, k is related (but not identical) to the ...