Chapter 5
... the spin quantum number (ms). E. none of these choices is correct 21. Atomic orbitals developed using quantum mechanics A. describe regions of space in which one is most likely to find an electron. B. describe exact paths for electron motion. C. give a description of the atomic structure which is es ...
... the spin quantum number (ms). E. none of these choices is correct 21. Atomic orbitals developed using quantum mechanics A. describe regions of space in which one is most likely to find an electron. B. describe exact paths for electron motion. C. give a description of the atomic structure which is es ...
Page 16(1)
... under continuous in time observation in the most general case of nondemolition measurement. 7.The special nondemolition measurement model of quantum stochastic calculus, the extended variant In this section we apply our results to the concrete special measurement model of quantum stochastic mechanic ...
... under continuous in time observation in the most general case of nondemolition measurement. 7.The special nondemolition measurement model of quantum stochastic calculus, the extended variant In this section we apply our results to the concrete special measurement model of quantum stochastic mechanic ...
Steve Hansen`s second test - Kwantlen Polytechnic University
... what would be its wavelength (in nanometers)? (4) ...
... what would be its wavelength (in nanometers)? (4) ...
Document
... Prediction – e– should emit light at whatever frequency f it orbits nucleus d sin θ = mλ ...
... Prediction – e– should emit light at whatever frequency f it orbits nucleus d sin θ = mλ ...
14. Multiple Particles
... particles are entangled. As the example above demonstrates, this means that you might be able to learn something about both particles by performing a measurement on just one of them. Wavefunctions typically become entangled when the particles interact with each other. For example, although the wavef ...
... particles are entangled. As the example above demonstrates, this means that you might be able to learn something about both particles by performing a measurement on just one of them. Wavefunctions typically become entangled when the particles interact with each other. For example, although the wavef ...
Mathematics of Quantum Mechanics
... force. The principles of Newtonian mechanics were proven to accurately describe the “laws of nature” in the macroscopic world. This was the philosophical outlook that pervaded Physics, in the years leading up to Schrodinger’s quantum mechanical wave equation, as the interpretations became more mathe ...
... force. The principles of Newtonian mechanics were proven to accurately describe the “laws of nature” in the macroscopic world. This was the philosophical outlook that pervaded Physics, in the years leading up to Schrodinger’s quantum mechanical wave equation, as the interpretations became more mathe ...
The Interaction of Radiation and Matter: Quantum
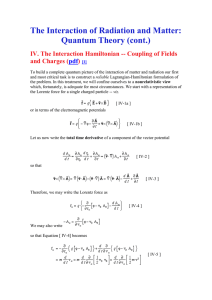
... IV. The Interaction Hamiltonian -- Coupling of Fields and Charges (pdf) [1] To build a complete quantum picture of the interaction of matter and radiation our first and most critical task is to construct a reliable Lagrangian-Hamiltonian formulation of the problem. In this treatment, we will confine ...
... IV. The Interaction Hamiltonian -- Coupling of Fields and Charges (pdf) [1] To build a complete quantum picture of the interaction of matter and radiation our first and most critical task is to construct a reliable Lagrangian-Hamiltonian formulation of the problem. In this treatment, we will confine ...
- Philsci
... If such free photons are involved, then (at least at the level of the system in the drawing) we don’t really have the light-tight box condition allowing for the use of D rather than DF. (In any case, D alone would not provide for the propagation of energy in only one direction; time-symmetric energy ...
... If such free photons are involved, then (at least at the level of the system in the drawing) we don’t really have the light-tight box condition allowing for the use of D rather than DF. (In any case, D alone would not provide for the propagation of energy in only one direction; time-symmetric energy ...
Basics Quantum Mechanics Prof. Ajoy Ghatak Department of
... The phenomenon of interference is based on the superposition principle according to which the resultant displacement at a particular point produced by a number of waves is the vector sum of the displacements produced by each one of the disturbances, so this principle is known as the superposition p ...
... The phenomenon of interference is based on the superposition principle according to which the resultant displacement at a particular point produced by a number of waves is the vector sum of the displacements produced by each one of the disturbances, so this principle is known as the superposition p ...
According to Newton`s ______ law, an object with no net force
... 1. (P4.1c) Give examples of the more precise scientific meaning than the meaning of work in everyday language. 2. (P4.1d) A hockey puck is sliding across the ice. A player exerts a constant force of 4.5 N over a distance of 0.15 m. How much work does the payer do on the puck? 3. (P4.1d) A 0.15 kg ba ...
... 1. (P4.1c) Give examples of the more precise scientific meaning than the meaning of work in everyday language. 2. (P4.1d) A hockey puck is sliding across the ice. A player exerts a constant force of 4.5 N over a distance of 0.15 m. How much work does the payer do on the puck? 3. (P4.1d) A 0.15 kg ba ...
Lecture 23
... Lasers work according to the same sort of idea. The idea is to use atoms or other quantum systems as a kind of circuit element. They are objects that can respond at optical frequencies, (which an LC circuit cannot!) The feedback is provided by the phenomenon of stimulated emission. Absorption, Spont ...
... Lasers work according to the same sort of idea. The idea is to use atoms or other quantum systems as a kind of circuit element. They are objects that can respond at optical frequencies, (which an LC circuit cannot!) The feedback is provided by the phenomenon of stimulated emission. Absorption, Spont ...
Questions
... Problem 3: Spectrum of Alkali Atoms (10 points) We have seen that the spectrum of hydrogen has an “accidental” degeneracy – states with the same principle quantum number n, but different angular momentum quantum number l are degenerate. This was an artifact of the pure 1/r Coulomb potential associa ...
... Problem 3: Spectrum of Alkali Atoms (10 points) We have seen that the spectrum of hydrogen has an “accidental” degeneracy – states with the same principle quantum number n, but different angular momentum quantum number l are degenerate. This was an artifact of the pure 1/r Coulomb potential associa ...
General Chemistry for Engineers
... By the beginning of the twentieth century Dmitri Mendeleev was able to organize elements in the form of a periodic table based on systematic trends in their properties. The periodic table arranges elements according to increasing atomic number in horizontal rows termed periods and vertical groups or ...
... By the beginning of the twentieth century Dmitri Mendeleev was able to organize elements in the form of a periodic table based on systematic trends in their properties. The periodic table arranges elements according to increasing atomic number in horizontal rows termed periods and vertical groups or ...
Pauli`s exclusion principle in spinor coordinate space
... usual formulation of quantum field theory. The structure of quantum mechanics itself is addressed, using differential geometry, in a way similar to general relativity. The resulting generalization of the Dirac equation allows the introduction of other properties that are know for electrons. It is an ...
... usual formulation of quantum field theory. The structure of quantum mechanics itself is addressed, using differential geometry, in a way similar to general relativity. The resulting generalization of the Dirac equation allows the introduction of other properties that are know for electrons. It is an ...
Chapter 41 Problems
... particle near L/2, by calculating the probability that the particle lies in the range 0.490L ≤ x ≤ 0.510L. (c) What If? Determine the probability of finding the particle near L/4, by calculating the probability that the particle lies in the range 0.240L ≤ x ≤ 0.260L. (d) Argue that the result of par ...
... particle near L/2, by calculating the probability that the particle lies in the range 0.490L ≤ x ≤ 0.510L. (c) What If? Determine the probability of finding the particle near L/4, by calculating the probability that the particle lies in the range 0.240L ≤ x ≤ 0.260L. (d) Argue that the result of par ...
Calculation of Hawking Radiation as Quantum Mechanical Tunneling
... A Fock basis for the g-observer can be constructed using this vacuum state and the creation operator just as well. In a general curved spacetime there is no reason to prefer one set of modes to any other. Every observer classifies modes to be positive- and negativefrequency with respect to his prope ...
... A Fock basis for the g-observer can be constructed using this vacuum state and the creation operator just as well. In a general curved spacetime there is no reason to prefer one set of modes to any other. Every observer classifies modes to be positive- and negativefrequency with respect to his prope ...
Full text in PDF form
... final, always subject to question and doubt. The scientific way of forming concepts differs from that which we use in our daily life, not basically, but merely in the more precise definition of concepts and conclusions; more painstaking and systematic choice of experimental material; and greater log ...
... final, always subject to question and doubt. The scientific way of forming concepts differs from that which we use in our daily life, not basically, but merely in the more precise definition of concepts and conclusions; more painstaking and systematic choice of experimental material; and greater log ...
SECTION B ( 48 marks )
... Which of the following statements is/are correct ? (1) The acceleration of the body is zero when it is at level X. (2) The strain energy of the spring is zero when the body is at level Y. (3) The net downward force acting on the body is at its maximum when it is at level Z A. (1) only B. (3) only C. ...
... Which of the following statements is/are correct ? (1) The acceleration of the body is zero when it is at level X. (2) The strain energy of the spring is zero when the body is at level Y. (3) The net downward force acting on the body is at its maximum when it is at level Z A. (1) only B. (3) only C. ...
Chemistry (CP) Final Exam Study Guide 1
... ____ 44. Using the periodic table, determine the number of neutrons in O. a. 4 c. 16 b. 8 d. 24 ____ 45. Which of the following statements is NOT true? a. Atoms of the same element can have different masses. b. Atoms of isotopes of an element have different numbers of protons. c. The nucleus of an ...
... ____ 44. Using the periodic table, determine the number of neutrons in O. a. 4 c. 16 b. 8 d. 24 ____ 45. Which of the following statements is NOT true? a. Atoms of the same element can have different masses. b. Atoms of isotopes of an element have different numbers of protons. c. The nucleus of an ...
Case Study 6
... The Discovery of the Atomic Nucleus The fact that the scattering law was obeyed so precisely, even for large angles of scattering, meant that the inverse-square law of electrostatic repulsion held good to very small distances indeed. The nucleus had to have size less than about 10−14 m, very much l ...
... The Discovery of the Atomic Nucleus The fact that the scattering law was obeyed so precisely, even for large angles of scattering, meant that the inverse-square law of electrostatic repulsion held good to very small distances indeed. The nucleus had to have size less than about 10−14 m, very much l ...