Atomic Structure: Chapter 5 Chapter Outline Chapter Outline
... • Light of a characteristic wavelength (and frequency) is absorbed when electron jumps from lower E (orbit, n = 2) to higher E (orbit, n= 4) • This is the origin of absorption spectra. ...
... • Light of a characteristic wavelength (and frequency) is absorbed when electron jumps from lower E (orbit, n = 2) to higher E (orbit, n= 4) • This is the origin of absorption spectra. ...
Set #4 - comsics
... Consider a positronium atom consisting of a positron and electron revolving about their common centre of mass, which lies halfway between them. (a) If such a system were a normal atom, how would its emission spectrum compared to that of hydrogen atom? (b) What would be the electron-positron separati ...
... Consider a positronium atom consisting of a positron and electron revolving about their common centre of mass, which lies halfway between them. (a) If such a system were a normal atom, how would its emission spectrum compared to that of hydrogen atom? (b) What would be the electron-positron separati ...
Document
... are with values of maverage =5.2V±0.7V and =237 nm±17 nm within the expected theoretical values of 4.9eV and 253.7nm. (iii) Measurements with the neon tube The second tube we examined was filled with neon which we did not need to heat because it is in the state of gas at room temperature. The proce ...
... are with values of maverage =5.2V±0.7V and =237 nm±17 nm within the expected theoretical values of 4.9eV and 253.7nm. (iii) Measurements with the neon tube The second tube we examined was filled with neon which we did not need to heat because it is in the state of gas at room temperature. The proce ...
Problems and Questions on Lecture 2 Useful equations and
... (A) positive charge and a mass approximately equal to a proton. (B) positive charge and a mass approximately equal to an electron. (C) neutral charge and a mass approximately equal to a proton. (D) neutral charge and a mass approximately equal to an electron. (E) negative charge and a mass approxima ...
... (A) positive charge and a mass approximately equal to a proton. (B) positive charge and a mass approximately equal to an electron. (C) neutral charge and a mass approximately equal to a proton. (D) neutral charge and a mass approximately equal to an electron. (E) negative charge and a mass approxima ...
Chapter 31 Atomic Physics
... associated energies have only discrete values, one uses integer numbers to identify each orbit and its corresponding energy. Such integer number is called a quantum number. Quantum mechanics describes the hydrogen atom in terms of four quantum numbers: (1) the principal quantum number n, which can h ...
... associated energies have only discrete values, one uses integer numbers to identify each orbit and its corresponding energy. Such integer number is called a quantum number. Quantum mechanics describes the hydrogen atom in terms of four quantum numbers: (1) the principal quantum number n, which can h ...
ELECTRON I: Free electron model
... occupy one state with the same energy, a state labled by quantum number n can accommodate two electrons. The Fermi energy, defined as the energy of the topmost filled state (relative to the energy of the ground state), can be calculated by counting the number of electrons in the system at zero tempe ...
... occupy one state with the same energy, a state labled by quantum number n can accommodate two electrons. The Fermi energy, defined as the energy of the topmost filled state (relative to the energy of the ground state), can be calculated by counting the number of electrons in the system at zero tempe ...
Physics 214b-2008 Walter F
... IMPORTANT: This exam will be truly cumulative, i.e. it will cover material from the entire semester. For example, it will cover material such as the quantum nature of light that we discussed back in chapter 1. However, there will be some extra emphasis on the material since exam 2, since you’ve not ...
... IMPORTANT: This exam will be truly cumulative, i.e. it will cover material from the entire semester. For example, it will cover material such as the quantum nature of light that we discussed back in chapter 1. However, there will be some extra emphasis on the material since exam 2, since you’ve not ...
Colloquium on "Many Worlds Interpretation"
... epistemological postulate of psycho-physical parallelism. In this interpretation, the physical world is completely described by Everett's wave function that evolves deterministically (Laplacean). This global quantum state then defines an indeterministic (hence "branching") succession of states for a ...
... epistemological postulate of psycho-physical parallelism. In this interpretation, the physical world is completely described by Everett's wave function that evolves deterministically (Laplacean). This global quantum state then defines an indeterministic (hence "branching") succession of states for a ...
Quantum Few-Body Systems
... the mechanism is very similar in both cases. We discuss the close relation between rotating BECs and quantum dots at strong magnetic fields. The vortices can stick to particles to form composite particles, but also occur without association to any particular particle. In quantum dots we find off-ele ...
... the mechanism is very similar in both cases. We discuss the close relation between rotating BECs and quantum dots at strong magnetic fields. The vortices can stick to particles to form composite particles, but also occur without association to any particular particle. In quantum dots we find off-ele ...
Word - Structured Independent Learning
... According to Einstein (1905), mass defect can be explained by assuming that an equivalent amount of energy is carried away by an energetic photon. The amount of energy equivalent to the mass defect could be predicted by the famous equation E = mc2. Assuming that the mass defect is 9.00 x 10-30 kg, t ...
... According to Einstein (1905), mass defect can be explained by assuming that an equivalent amount of energy is carried away by an energetic photon. The amount of energy equivalent to the mass defect could be predicted by the famous equation E = mc2. Assuming that the mass defect is 9.00 x 10-30 kg, t ...
Electrons in the Atom
... So scientists agreed to limit these calculations to locations where there was at least a 90% chance of finding an electron. Think of orbitals as sort of a "border” for spaces around the nucleus inside which electrons are allowed. No more than 2 electrons can ever be in 1 orbital. The orbital j ...
... So scientists agreed to limit these calculations to locations where there was at least a 90% chance of finding an electron. Think of orbitals as sort of a "border” for spaces around the nucleus inside which electrons are allowed. No more than 2 electrons can ever be in 1 orbital. The orbital j ...
Lect 23 Presentation
... The electrons in a large group of hydrogen atoms are excited to the n=3 level. How many spectral lines will be produced? ...
... The electrons in a large group of hydrogen atoms are excited to the n=3 level. How many spectral lines will be produced? ...
Physics 452 - BYU Physics and Astronomy
... Consider 3 distinguishable particles in a harmonic oscillator potential. If the total energy of the system is E 9 / 2 ...
... Consider 3 distinguishable particles in a harmonic oscillator potential. If the total energy of the system is E 9 / 2 ...
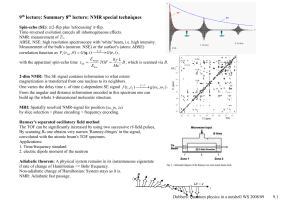
Δk/k
... ic1(1) c1(0)V11 c2( 0)V12 e iω0t 0 0 0 , that is c1(1) remains unchanged: c1(1) (t ) 1 , and the probability amplitude c2 for transition between the states is E2 ic2(1) c2( 0)V22 c1(0)V21e iω0t 0 12 ω1 (t )e iω0t , ...
... ic1(1) c1(0)V11 c2( 0)V12 e iω0t 0 0 0 , that is c1(1) remains unchanged: c1(1) (t ) 1 , and the probability amplitude c2 for transition between the states is E2 ic2(1) c2( 0)V22 c1(0)V21e iω0t 0 12 ω1 (t )e iω0t , ...