Topic 2 IB Chemistry Assessment Statements 2009 Revised File
... and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of quantum numbers and historical references w ...
... and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of quantum numbers and historical references w ...
Variational principle - Indiana University Bloomington
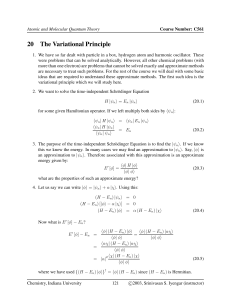
... 1. We have so far dealt with particle in a box, hydrogen atom and harmonic oscillator. These were problems that can be solved analytically. However, all other chemical problems (with more than one electron) are problems that cannot be solved exactly and approximate methods are necessary to treat suc ...
... 1. We have so far dealt with particle in a box, hydrogen atom and harmonic oscillator. These were problems that can be solved analytically. However, all other chemical problems (with more than one electron) are problems that cannot be solved exactly and approximate methods are necessary to treat suc ...
Flame Test Lab
... energy level is available, the electron will “fall” back, giving off energy in the form of electromagnetic radiation (light) in the process. The difference in energies between the two levels is emitted in the form of a photon (aka “quantum”) of electromagnetic radiation. The energy of each photon is ...
... energy level is available, the electron will “fall” back, giving off energy in the form of electromagnetic radiation (light) in the process. The difference in energies between the two levels is emitted in the form of a photon (aka “quantum”) of electromagnetic radiation. The energy of each photon is ...
Electrons
... • Electrons can absorb varying sized quanta and “jump” to varying excited states • They also “drop” from excited state to ground state and release a different amount of energy and a different color of light • In any given sample of an element, all possible jumps and drops are taking place • Not all ...
... • Electrons can absorb varying sized quanta and “jump” to varying excited states • They also “drop” from excited state to ground state and release a different amount of energy and a different color of light • In any given sample of an element, all possible jumps and drops are taking place • Not all ...
Exploring physics capabilities in the STAR experiment with the
... Whether AA creates a medium long-lived and extend over sizable volume and reached the thermodynamics limit to have particular thermodynamic and transport properties.?! ...
... Whether AA creates a medium long-lived and extend over sizable volume and reached the thermodynamics limit to have particular thermodynamic and transport properties.?! ...
Lecture 11
... • The electron in the H atom can go from one shell to a lower one by emitting a photon. The series of transitions from principal number n ≥ 2 to n = 1 is called the Lyman series 2 . The transitions are names by Greek letters: the transition from n = 2 to n = 1 is called Lyman-α, from 3 to 1 Lyman-β, ...
... • The electron in the H atom can go from one shell to a lower one by emitting a photon. The series of transitions from principal number n ≥ 2 to n = 1 is called the Lyman series 2 . The transitions are names by Greek letters: the transition from n = 2 to n = 1 is called Lyman-α, from 3 to 1 Lyman-β, ...
High School Physical Science Glossary
... subshell- orbitals of various shapes and energies within an energy level; which are s,p,d,f synthesis reactions- chemical reaction in which a single compound is created from two or more reactants (A + B AB) temperature- measure of the average kinetic energy of the particles within a substance test ...
... subshell- orbitals of various shapes and energies within an energy level; which are s,p,d,f synthesis reactions- chemical reaction in which a single compound is created from two or more reactants (A + B AB) temperature- measure of the average kinetic energy of the particles within a substance test ...
5-11_Stuewer
... produced by light, without assuming that light is made up of unalterable units, each containing a definite and, on Planck's hypothesis, a comparatively large amount of energy, a view which it is exceedingly difficult to reconcile with well-known optical phenomena." 24 J. H. Jeans criticized25 Thomso ...
... produced by light, without assuming that light is made up of unalterable units, each containing a definite and, on Planck's hypothesis, a comparatively large amount of energy, a view which it is exceedingly difficult to reconcile with well-known optical phenomena." 24 J. H. Jeans criticized25 Thomso ...
Ladder Operators
... of solving the TISE for the simple harmonic oscillator. The bad news, though, is that no such elegant method exists for solving the TISE for other one-dimensional potential functions; the method worked here only because the Hamiltonian is quadratic in both p and x, allowing it to be factored, aside ...
... of solving the TISE for the simple harmonic oscillator. The bad news, though, is that no such elegant method exists for solving the TISE for other one-dimensional potential functions; the method worked here only because the Hamiltonian is quadratic in both p and x, allowing it to be factored, aside ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... Let rot be the frequency of rotations (Cycles/second) The velocity of particle v=2πrrot= r ωrot where ωrot=2πrot has units of radians/second and is called the angular velocity. The kinetic energy of the revolving particle is: ...
... Let rot be the frequency of rotations (Cycles/second) The velocity of particle v=2πrrot= r ωrot where ωrot=2πrot has units of radians/second and is called the angular velocity. The kinetic energy of the revolving particle is: ...
Chapter 5 Electrons in Atoms
... to move from one energy level to another. Since the energy of an atom is never “in between” there must be a quantum leap in energy. ...
... to move from one energy level to another. Since the energy of an atom is never “in between” there must be a quantum leap in energy. ...
1 pint
... Trygve Helgaker and Igor Tomashevsky* Department of Chemistry, University of Oslo, Blindem, N-0315Oslo 3, Norway ...
... Trygve Helgaker and Igor Tomashevsky* Department of Chemistry, University of Oslo, Blindem, N-0315Oslo 3, Norway ...
Full text in PDF form
... quantum mechanics. Thereby we focus on the reconstruction of the most simple situation where there is a free particle in space of temperature T . Due to the probabilistic nature of quantum mechanics it is clear that the reconstruction can only be done by using quantities which are at most analogous ...
... quantum mechanics. Thereby we focus on the reconstruction of the most simple situation where there is a free particle in space of temperature T . Due to the probabilistic nature of quantum mechanics it is clear that the reconstruction can only be done by using quantities which are at most analogous ...