CONJECTURING THE MATHEMATICAL AXIOM THAT
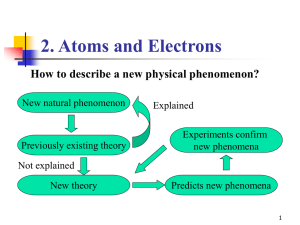
... been ignored, but it has been neglected. In quantum physics, it has been unjustly neglected. One usually considers situations that are too idealized, and one investigates problems for which the directedness of time and for which irreversibility do not play a prominent role. An example is classical m ...
... been ignored, but it has been neglected. In quantum physics, it has been unjustly neglected. One usually considers situations that are too idealized, and one investigates problems for which the directedness of time and for which irreversibility do not play a prominent role. An example is classical m ...
Quantum Mechanics as dissolver of the sensate universe: this is
... Louis de Brogli generalized wave particle duality by associating a wave length not only with mass-less photons, but also electrons, and in fact to any material body. Validity of the de Broglie hypothesis has been confirmed for macromolecules, as well as molecules, atoms, and subatomic particles. 1 M ...
... Louis de Brogli generalized wave particle duality by associating a wave length not only with mass-less photons, but also electrons, and in fact to any material body. Validity of the de Broglie hypothesis has been confirmed for macromolecules, as well as molecules, atoms, and subatomic particles. 1 M ...
Eighth International Conference on Geometry, Integrability and Quantization
... In the nineteen fifties of previous century, Destouches [1, 2] developed his functional quantum theory as a generalization of de Broglie’s theory. His basic idea was that elementary particles need not be pointlike. Being extended and non rigid is a better conception. Rather than conceiving the parti ...
... In the nineteen fifties of previous century, Destouches [1, 2] developed his functional quantum theory as a generalization of de Broglie’s theory. His basic idea was that elementary particles need not be pointlike. Being extended and non rigid is a better conception. Rather than conceiving the parti ...
influências da expansão do universo na evolução do - Cosmo-ufes
... wave function for the quantum part, and classical variables -variables which have values - for the classical part: (Ψ(t,q ...), X(t) ...). The Xs are somehow macroscopic. This is not spelled out very explicitly. The dynamics is not very precisely formulated either. It includes a Schrödinger equation ...
... wave function for the quantum part, and classical variables -variables which have values - for the classical part: (Ψ(t,q ...), X(t) ...). The Xs are somehow macroscopic. This is not spelled out very explicitly. The dynamics is not very precisely formulated either. It includes a Schrödinger equation ...
Chemistry 681 Introduction to Quantum
... Chemistry 681 Introduction to Quantum Chemistry Fall 2003 ...
... Chemistry 681 Introduction to Quantum Chemistry Fall 2003 ...
poster
... itself, and then collapses to a point upon detection. Copenhagen/Agnostic (C/A): Electrons are modeled in terms of waves or particles accordingly; emphasis on mathematical calculation (predicting features of the interference pattern) over interpretation. ...
... itself, and then collapses to a point upon detection. Copenhagen/Agnostic (C/A): Electrons are modeled in terms of waves or particles accordingly; emphasis on mathematical calculation (predicting features of the interference pattern) over interpretation. ...
File - SPHS Devil Physics
... a. Aim 1: study of quantum phenomena introduces students to an exciting new world that is not experienced at the macroscopic level. The study of tunneling is a novel phenomenon not observed in macroscopic physics. b. Aim 6: the photoelectric effect can be investigated using LEDs c. Aim 9: the Bohr m ...
... a. Aim 1: study of quantum phenomena introduces students to an exciting new world that is not experienced at the macroscopic level. The study of tunneling is a novel phenomenon not observed in macroscopic physics. b. Aim 6: the photoelectric effect can be investigated using LEDs c. Aim 9: the Bohr m ...
“Can Quantum-Mechanical Description of Physical Reality Be Considered Complete?” JOSEPH LEONARD TUBERGEN
... “Can Quantum-Mechanical Description of Physical Reality Be Considered Complete?” JOSEPH LEONARD TUBERGEN Physics Major, University of NC Wilmington Erwin with his psi can do Calculations quite a few. But one thing has not been seen: Just what does psi really mean? -Erich Hückel This was the title of ...
... “Can Quantum-Mechanical Description of Physical Reality Be Considered Complete?” JOSEPH LEONARD TUBERGEN Physics Major, University of NC Wilmington Erwin with his psi can do Calculations quite a few. But one thing has not been seen: Just what does psi really mean? -Erich Hückel This was the title of ...
File
... Niels Bohr (1885–1962) Bohr reconciled Rutherford’s results from the gold foil experiment with Planck’s quantum theory to create a model of the atom in which electrons resided in specific energy levels at specific stable radii. This model was the basis for Balmer’s work with spectroscopy and Rydberg ...
... Niels Bohr (1885–1962) Bohr reconciled Rutherford’s results from the gold foil experiment with Planck’s quantum theory to create a model of the atom in which electrons resided in specific energy levels at specific stable radii. This model was the basis for Balmer’s work with spectroscopy and Rydberg ...
Department of Physics and Physical Oceanography Sigma Pi Sigma INDUCTION
... Department of Physics, Yeshiva University ...
... Department of Physics, Yeshiva University ...
Periodic boundary physics etc
... In physics, specifically quantum mechanics, the Schrödinger equation is an equation that describes how the quantum state of a physical system changes in time. It is as central to quantum mechanics as Newton's laws are to classical mechanics. In the standard interpretation of quantum mechanics, the q ...
... In physics, specifically quantum mechanics, the Schrödinger equation is an equation that describes how the quantum state of a physical system changes in time. It is as central to quantum mechanics as Newton's laws are to classical mechanics. In the standard interpretation of quantum mechanics, the q ...
Lecture Notes, Feb 29
... looked at de Broglie’s thesis. He worked out a single equation, explaining the behavior of particles in terms of de Broglie waves. The lead player in the equation is a quantity called Ψ ( pronounced ”sigh” ) which is called the wave function. • Instead of describing particle by its position and velo ...
... looked at de Broglie’s thesis. He worked out a single equation, explaining the behavior of particles in terms of de Broglie waves. The lead player in the equation is a quantity called Ψ ( pronounced ”sigh” ) which is called the wave function. • Instead of describing particle by its position and velo ...
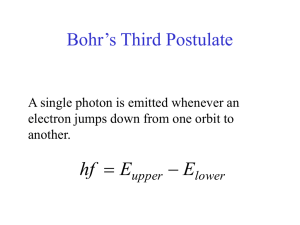
Bohr´s Third Postulate
... If we are dealing only with one photon: At any point the square of the electric field strength is a measure of the probability that a photon will be at that location. ...
... If we are dealing only with one photon: At any point the square of the electric field strength is a measure of the probability that a photon will be at that location. ...
The mathematical formulations of quantum mechanics are those
... not be predicted. The mathematical status of quantum theory remained uncertain for some time. In 1923 de Broglie proposed that wave-particle duality applied not only to photons but to electrons and every other physical system. The situation changed rapidly in the years 1925–1930, when working mathem ...
... not be predicted. The mathematical status of quantum theory remained uncertain for some time. In 1923 de Broglie proposed that wave-particle duality applied not only to photons but to electrons and every other physical system. The situation changed rapidly in the years 1925–1930, when working mathem ...
PHYS6520 Quantum Mechanics II Spring 2013 HW #5
... (d) Confirm that you get the same result by using grade-school quantum mechanics and matching right and left going waves on the left with a right going wave on the right at x = 0. You’ll need to integrate the Schrödinger equation across x = 0 to match the derivatives. (e) We showed last semester th ...
... (d) Confirm that you get the same result by using grade-school quantum mechanics and matching right and left going waves on the left with a right going wave on the right at x = 0. You’ll need to integrate the Schrödinger equation across x = 0 to match the derivatives. (e) We showed last semester th ...
Postulate 1 of Quantum Mechanics (wave function)
... http://vergil.chemistry.gatech.edu/notes/quantrev/quantrev.html • Wikipedia (http://en.wikipedia.org): Search for Wave function Measurement in quantum mechanics Schrodinger equation ...
... http://vergil.chemistry.gatech.edu/notes/quantrev/quantrev.html • Wikipedia (http://en.wikipedia.org): Search for Wave function Measurement in quantum mechanics Schrodinger equation ...