12.3 Assembly of distinguishable Particles
... highest probability. • The macrostate with the highest thermodynamic probability will be the observed equilibrium state of the system. • The statistical model suggests that systems tend to change spontaneously from states with low thermodynamic probability to states with high thermodynamic probabili ...
... highest probability. • The macrostate with the highest thermodynamic probability will be the observed equilibrium state of the system. • The statistical model suggests that systems tend to change spontaneously from states with low thermodynamic probability to states with high thermodynamic probabili ...
Boltzmann factors and partition functions revisited
... Distinguishable means that, although the particles have the same chemical identity, they are localized in distinct regions of space and are (in principle) experimentally identifiable (“taggable”) according to their location. (Example: molecules fixed at distinct sites in a crystal lattice.) From (16 ...
... Distinguishable means that, although the particles have the same chemical identity, they are localized in distinct regions of space and are (in principle) experimentally identifiable (“taggable”) according to their location. (Example: molecules fixed at distinct sites in a crystal lattice.) From (16 ...
JCE0597 p605 Numerical Methods for Finding Momentum Space
... first direct experimental confirmation of a state predicted by Einstein in 1925) of several thousand rubidium atoms confined to the ground state of a three-dimensional harmonic potential well. In such a condensate the rubidium atoms are all in the same quantum state and as such represent the materia ...
... first direct experimental confirmation of a state predicted by Einstein in 1925) of several thousand rubidium atoms confined to the ground state of a three-dimensional harmonic potential well. In such a condensate the rubidium atoms are all in the same quantum state and as such represent the materia ...
Notes for Lecture 2 Miller Indices, Quantum Mechanics
... To understand this figure, imagine that the wave of an electron in a hydrogen atom is like a wave of a guitar string. Except that the guitar string is a circular string. Imagine that you pick one part of the string (S in the figure). When you do that, a wave propagates in two directions (the red dir ...
... To understand this figure, imagine that the wave of an electron in a hydrogen atom is like a wave of a guitar string. Except that the guitar string is a circular string. Imagine that you pick one part of the string (S in the figure). When you do that, a wave propagates in two directions (the red dir ...
Particle in a box (PPT - 6.9MB)
... In 1877 he went to Berlin for a year of study with physicists Helmholtz and Kirchhoff. He wrote that Kirchhoff spoke in carefully prepared lectures which were dry and monotonous. He eventually became Kirchhoff’s successor in Berlin. The concept of the photon was initially rejected by Planck. He wrot ...
... In 1877 he went to Berlin for a year of study with physicists Helmholtz and Kirchhoff. He wrote that Kirchhoff spoke in carefully prepared lectures which were dry and monotonous. He eventually became Kirchhoff’s successor in Berlin. The concept of the photon was initially rejected by Planck. He wrot ...
Particle in a box - MIT OpenCourseWare
... The concept of the photon was initially rejected by Planck. He wrote "The theory of light would be thrown back not by decades, but by centuries, into the age when Christian Huygens dared to fight against the mighty emission theory of Isaac Newton.“ In his Scientific Autobiography and Other Papers, h ...
... The concept of the photon was initially rejected by Planck. He wrote "The theory of light would be thrown back not by decades, but by centuries, into the age when Christian Huygens dared to fight against the mighty emission theory of Isaac Newton.“ In his Scientific Autobiography and Other Papers, h ...
chapter 4
... At the source the electron is being emitted as particle and is experimentally detected as a electron which is absorbed by an individual atom in the fluorescent plate In between, we must interpret the electron in the form of a wave. The double slits change the propagation of the electron wave so that ...
... At the source the electron is being emitted as particle and is experimentally detected as a electron which is absorbed by an individual atom in the fluorescent plate In between, we must interpret the electron in the form of a wave. The double slits change the propagation of the electron wave so that ...
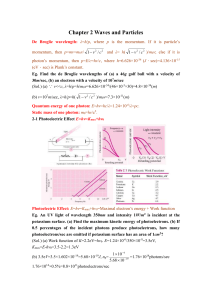
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
... potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2? (Sol.) (a) Work function of K=2.2eV=hν0. E=1.24×10-6/350×10-9=3.5eV, ...
... potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2? (Sol.) (a) Work function of K=2.2eV=hν0. E=1.24×10-6/350×10-9=3.5eV, ...
Lecture Notes (pptx) - Cornell Computer Science
... Science fiction writers imagine that quantum computing (or some other form of physical computing) might somehow break all classical limits This seems not to be possible, but we could be wrong. After all, we’ve only been in this business for a few years… Right now, quantum computing may be most usefu ...
... Science fiction writers imagine that quantum computing (or some other form of physical computing) might somehow break all classical limits This seems not to be possible, but we could be wrong. After all, we’ve only been in this business for a few years… Right now, quantum computing may be most usefu ...
DeBroglie Hypothesis
... Heisenberg Uncertainty Principle b) A particle does have a definite location at a specific time, but it does not have a frequency or wavelength. c) Inbetween case: a group of sine waves can add together (via Fourier analysis) to give a semi-definite location: a result of Fourier analysis is this: t ...
... Heisenberg Uncertainty Principle b) A particle does have a definite location at a specific time, but it does not have a frequency or wavelength. c) Inbetween case: a group of sine waves can add together (via Fourier analysis) to give a semi-definite location: a result of Fourier analysis is this: t ...
LECTURE 18
... •We can find allowed energy levels by plugging those wavefunctions into the Schrodinger equation and solving for the energy. •We know that the particle’s position cannot be determined precisely, but that the probability of a particle being found at a particular point can be calculated from the wave- ...
... •We can find allowed energy levels by plugging those wavefunctions into the Schrodinger equation and solving for the energy. •We know that the particle’s position cannot be determined precisely, but that the probability of a particle being found at a particular point can be calculated from the wave- ...
Chap 3.
... to right on the x-axis, with momentum p > 0. Correspondingly, e−ikx represents motion from right to left with p < 0. The functions sin kx and cos kx represent standing waves, obtained by superposition of opposing wave motions. Although these latter two are not eigenfunctions of p̂x but are eigenfunc ...
... to right on the x-axis, with momentum p > 0. Correspondingly, e−ikx represents motion from right to left with p < 0. The functions sin kx and cos kx represent standing waves, obtained by superposition of opposing wave motions. Although these latter two are not eigenfunctions of p̂x but are eigenfunc ...
Summary of Important Ideas in Quantum Physics
... An -particle consists of two protons and two neutrons. (This is a helium nucleus.) -decay proceeds via the mechanism described in Item (6). A -particle is a high-energy electron. In some barely-bound nuclei, a second, much weaker nuclear force known as the weak force can compete with the strong ...
... An -particle consists of two protons and two neutrons. (This is a helium nucleus.) -decay proceeds via the mechanism described in Item (6). A -particle is a high-energy electron. In some barely-bound nuclei, a second, much weaker nuclear force known as the weak force can compete with the strong ...
PHYS150-Ch27
... The Thomson model of the atom had a volume of positive charge with the negatively charged electrons embedded within the volume. ScaGering experiments by Rutherford led to the conclusion that an atom had a very small nucleus of positive charge (10-‐‑5 ti ...
... The Thomson model of the atom had a volume of positive charge with the negatively charged electrons embedded within the volume. ScaGering experiments by Rutherford led to the conclusion that an atom had a very small nucleus of positive charge (10-‐‑5 ti ...
Quantum Interference 3 Claude Cohen-Tannoudji Scott Lectures Cambridge, March 9
... of the particle, the 2 states E+ and E- must be clearly distinct without any overlap. Their scalar product must be equal to 0 so that the fringes vanish This result can be extended to any quantum device which could be introduced for determining the path of the atom. If the device is efficient, i.e. ...
... of the particle, the 2 states E+ and E- must be clearly distinct without any overlap. Their scalar product must be equal to 0 so that the fringes vanish This result can be extended to any quantum device which could be introduced for determining the path of the atom. If the device is efficient, i.e. ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.