PRACTICE EXAM 1-C
... Potassium levels in the blood are measured in milliequivalents (mEq) of potassium per liter of blood, or “mEq/L”. A normal-range potassium level is around 4.3 mEq/L. Determine the mass of potassium contained in 1.00 pint of blood with a potassium level of 4.3 mEq/L. (7 pts) ...
... Potassium levels in the blood are measured in milliequivalents (mEq) of potassium per liter of blood, or “mEq/L”. A normal-range potassium level is around 4.3 mEq/L. Determine the mass of potassium contained in 1.00 pint of blood with a potassium level of 4.3 mEq/L. (7 pts) ...
Vitamins
... 4. Then titrate with the standard potassium iodate (KIO3) solution until the solution turns intense blue. Write down the standard potassium iodate (KIO3) solution volume. 5. Pipette 25 ml of an unknown ascorbic acid sample, a kind of juice, into a 250 ml conical flask, then follow the same procedure ...
... 4. Then titrate with the standard potassium iodate (KIO3) solution until the solution turns intense blue. Write down the standard potassium iodate (KIO3) solution volume. 5. Pipette 25 ml of an unknown ascorbic acid sample, a kind of juice, into a 250 ml conical flask, then follow the same procedure ...
KLOR-CON® M (Potassium Chloride Extended - Upsher
... The potassium ion is the principal intracellular cation of most body tissues. Potassium ions participate in a number of essential physiological processes including the maintenance of intracellular tonicity; the transmission of nerve impulses; the contraction of cardiac, skeletal, and smooth muscle; ...
... The potassium ion is the principal intracellular cation of most body tissues. Potassium ions participate in a number of essential physiological processes including the maintenance of intracellular tonicity; the transmission of nerve impulses; the contraction of cardiac, skeletal, and smooth muscle; ...
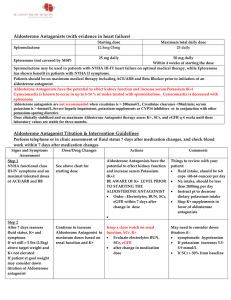
Aldosterone Antagonists
... Within 4 weeks of starting the dose Spironolactone may be used in patients with NYHA III‐IV heart failure on optimal medical therapy, while Eplerenone has shown benefit in patients with NYHA II symptoms. Patients should be on maximum medical therapy including ACE/ARB and Beta Blocker prior to in ...
... Within 4 weeks of starting the dose Spironolactone may be used in patients with NYHA III‐IV heart failure on optimal medical therapy, while Eplerenone has shown benefit in patients with NYHA II symptoms. Patients should be on maximum medical therapy including ACE/ARB and Beta Blocker prior to in ...
Answers - U of L Class Index
... lithium)? As part of your answer, write a balanced chemical equation (including states of matter) describing the industrial process used to make potassium metal. [2 marks] KCl(l) + Na(l) → K(g) + NaCl(l) Potassium is made by chemical reduction of the K+ in KCl(l) using sodium metal as the reducing a ...
... lithium)? As part of your answer, write a balanced chemical equation (including states of matter) describing the industrial process used to make potassium metal. [2 marks] KCl(l) + Na(l) → K(g) + NaCl(l) Potassium is made by chemical reduction of the K+ in KCl(l) using sodium metal as the reducing a ...
Mr. B`s Chemistry
... Write formulas for the reactants and predicted products for the chemical reactions that follow. Assume that in all cases a reaction occurs. The equation must be balanced. Write all substances in their proper form –as ions if appropriate – and cancel any spectator ions. ...
... Write formulas for the reactants and predicted products for the chemical reactions that follow. Assume that in all cases a reaction occurs. The equation must be balanced. Write all substances in their proper form –as ions if appropriate – and cancel any spectator ions. ...
this document in Microsoft Word format
... very different, and the US Food and Drug Administration has expressed serious concerns about the safety and effectiveness of iodate, and the fact that its manufacturers are not in conformity with FDA rules to assure safety, quality and purity of the product. Although the FDA has been successful at r ...
... very different, and the US Food and Drug Administration has expressed serious concerns about the safety and effectiveness of iodate, and the fact that its manufacturers are not in conformity with FDA rules to assure safety, quality and purity of the product. Although the FDA has been successful at r ...
Lecture 11 - U of L Class Index
... heated. Melting points of most Group 2 compounds are very high, due to the strong attractive forces between the M2+ cation with the anions present (page 119). For example, CaO melts at 2572°C, a temperature well beyond the range of an ordinary fire. Calcium compounds such as lime (CaO) were known an ...
... heated. Melting points of most Group 2 compounds are very high, due to the strong attractive forces between the M2+ cation with the anions present (page 119). For example, CaO melts at 2572°C, a temperature well beyond the range of an ordinary fire. Calcium compounds such as lime (CaO) were known an ...
Frequently Asked Questions on Potassium Iodide (KI)
... the recommended dose, KI is effective in reducing the risk of thyroid cancer in individuals or populations at risk for inhalation or ingestion of radioiodines. KI floods the thyroid with nonradioactive iodine and prevents the uptake of the radioactive molecules, which are subsequently excreted in ...
... the recommended dose, KI is effective in reducing the risk of thyroid cancer in individuals or populations at risk for inhalation or ingestion of radioiodines. KI floods the thyroid with nonradioactive iodine and prevents the uptake of the radioactive molecules, which are subsequently excreted in ...
8178 POTASSIUM NITRITE (English) ANACHEMIA MSDS
... materials. Do not add any other material to the container. Do not wash down the drain. Do not breathe dust. Keep away from direct sunlight or strong incandescent light. Keep container tightly closed and dry. Manipulate under an adequate fume hood. Avoid raising dust. Product is highly hygroscopic. U ...
... materials. Do not add any other material to the container. Do not wash down the drain. Do not breathe dust. Keep away from direct sunlight or strong incandescent light. Keep container tightly closed and dry. Manipulate under an adequate fume hood. Avoid raising dust. Product is highly hygroscopic. U ...
Lecture 25 Other Phosphate Fertilizers – Part 1
... for plants and formerly in animal feed. As a slow-release fertilizer, bone meal is primarily used as a source of phosphorus. As a fertilizer, the N-P-K ratio of bone meal is generally 4-12-0, though some steamed bone meals have N-P-Ks of 1-13-0. Bone meal is also an excellent organic source of calci ...
... for plants and formerly in animal feed. As a slow-release fertilizer, bone meal is primarily used as a source of phosphorus. As a fertilizer, the N-P-K ratio of bone meal is generally 4-12-0, though some steamed bone meals have N-P-Ks of 1-13-0. Bone meal is also an excellent organic source of calci ...
POTASSIUM SUPPLEMENTS (parenteral) - DavisPlus
... should be corrected to facilitate effectiveness of potassium replacement. Monitor serum chloride because hypochloremia may occur if replacing potassium without concurrent chloride. ● Toxicity and Overdose: Symptoms of toxicity are those of hyperkalemia (slow, irregular heartbeat; fatigue; muscle wea ...
... should be corrected to facilitate effectiveness of potassium replacement. Monitor serum chloride because hypochloremia may occur if replacing potassium without concurrent chloride. ● Toxicity and Overdose: Symptoms of toxicity are those of hyperkalemia (slow, irregular heartbeat; fatigue; muscle wea ...
potassium acid phosphate - DavisPlus
... complexes, loss of P waves, and cardiac arrhythmias) and hyperphosphatemia or hypocalcemia (paresthesia, muscle twitching, laryngospasm, colic, cardiac arrhythmias, or Chvostek’s or Trousseau’s sign). ● Treatment includes discontinuation of infusion, calcium replacement, and lowering serum potassium ...
... complexes, loss of P waves, and cardiac arrhythmias) and hyperphosphatemia or hypocalcemia (paresthesia, muscle twitching, laryngospasm, colic, cardiac arrhythmias, or Chvostek’s or Trousseau’s sign). ● Treatment includes discontinuation of infusion, calcium replacement, and lowering serum potassium ...
Potassium Bromide Oral Suspension
... missed doses and ask if an increase in the dose for a few days might be required. Dosing This medication has a 24 day half-life and it may take three to four months before seizure control occurs. This lengthy time line can be decreased by the administration of a loading dose. A loading dose is a hig ...
... missed doses and ask if an increase in the dose for a few days might be required. Dosing This medication has a 24 day half-life and it may take three to four months before seizure control occurs. This lengthy time line can be decreased by the administration of a loading dose. A loading dose is a hig ...
Veltassa ® - Patiromer Literature Review
... Weir, MR et al. N Engl J Med. 2015;Vol372:Pages 211-21. ...
... Weir, MR et al. N Engl J Med. 2015;Vol372:Pages 211-21. ...
What is potassium and what does it do in the
... What is potassium and what does it do in the body? Potassium is a mineral that controls nerve and muscle function. One very important muscle—the heart—beats at a normal rhythm because of potassium. In addition potassium is necessary for maintaining fluid and electrolyte balance and pH level. ...
... What is potassium and what does it do in the body? Potassium is a mineral that controls nerve and muscle function. One very important muscle—the heart—beats at a normal rhythm because of potassium. In addition potassium is necessary for maintaining fluid and electrolyte balance and pH level. ...
Potassium - HPS Chapters
... cells. Potassium is one of the most reactive metals in nature, and it forms a number of compounds that have many more commercial uses. For example, the white solid potassium bromide is used in photography, engraving, and lithography. The red crystal potassium chromate and yellow crystal potassium bi ...
... cells. Potassium is one of the most reactive metals in nature, and it forms a number of compounds that have many more commercial uses. For example, the white solid potassium bromide is used in photography, engraving, and lithography. The red crystal potassium chromate and yellow crystal potassium bi ...
- 5`d*
... how are atoms A and B related to one another? a. Atoms A and B are isotopes ."' bi. Atoms A and B are atoms of different elements \--c. All of the above are correct ...
... how are atoms A and B related to one another? a. Atoms A and B are isotopes ."' bi. Atoms A and B are atoms of different elements \--c. All of the above are correct ...
resonium a - product information
... Cation donating agents may reduce the effectiveness of the resin in binding potassium. Non-absorbable cation containing antacids and laxatives such as magnesium hydroxide and concomitant oral use of cation exchange resins has been reported to cause systemic alkalosis. Aluminium hydroxide: inte ...
... Cation donating agents may reduce the effectiveness of the resin in binding potassium. Non-absorbable cation containing antacids and laxatives such as magnesium hydroxide and concomitant oral use of cation exchange resins has been reported to cause systemic alkalosis. Aluminium hydroxide: inte ...
clin sys MENU v 8
... ELITech Clinical Systems offers a broad and growing menu of liquid-stable reagents backed by more than 25 years of experience in assay development and reagent manufacturing. ELITech reagents are: ...
... ELITech Clinical Systems offers a broad and growing menu of liquid-stable reagents backed by more than 25 years of experience in assay development and reagent manufacturing. ELITech reagents are: ...
Perchloracap - Nuclear Education Online
... females were given daily doses at 18 and 130 times the recommended human dose, respectively. In rabbits, potassium perchlorate was given daily throughout pregnancy at 100 mg/kg for 28 days. In guinea pigs, the mean daily dose was 740 mg/kg during the latter half of pregnancy, with an average treatme ...
... females were given daily doses at 18 and 130 times the recommended human dose, respectively. In rabbits, potassium perchlorate was given daily throughout pregnancy at 100 mg/kg for 28 days. In guinea pigs, the mean daily dose was 740 mg/kg during the latter half of pregnancy, with an average treatme ...
potassium and sodium phosphates - DavisPlus
... Treatment and prevention of phosphate depletion in patients who are unable to ingest adequate dietary phosphate. Adjunct therapy of urinary tract infections with methenamine hippurate or mandelate. Prevention of calcium urinary stones. Phosphate salts of potassium may be used in hypokalemic patients ...
... Treatment and prevention of phosphate depletion in patients who are unable to ingest adequate dietary phosphate. Adjunct therapy of urinary tract infections with methenamine hippurate or mandelate. Prevention of calcium urinary stones. Phosphate salts of potassium may be used in hypokalemic patients ...
Potassium

Potassium is a chemical element with symbol K (derived from Neo-Latin kalium) and atomic number 19. It was first isolated from potash, the ashes of plants, from which its name is derived. In the Periodic table, potassium is one of seven elements in column (group) 1 (alkali metals): they all have a single electron in their outer electron shell, which they readily give up to create an atom with a positive charge - a cation, and combine with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction and burning with a lilac flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and is part of many minerals. Naturally occurring potassium is composed of three isotopes, one of which, 40K, is radioactive. Traces of 40K are found in all potassium, and it is the most common radioisotope in the human body.Potassium is chemically very similar to sodium, which is also in column 1 of the Periodic table, and is in row 3, adjacent to potassium in row 4. They both have a similar ionization energy, which allows for each atom to give up its sole outer electron. The fact that they are different elements, each combining with the same anions to make similar salts, was suspected in 1702, and was proven in 1807 using electrolysis.Most industrial applications of potassium exploit the high solubility in water of potassium compounds, such as potassium soaps. Heavy crop production rapidly depletes soils of potassium, and this depletion is prevented and remedied with agricultural fertilizers containing potassium, which account for 95% of global potassium chemical production.Potassium ions are necessary for the function of all living cells. Potassium ion shifts across nerve cell membranes are necessary for normal nerve transmission: potassium depletion or excess can result in numerous abnormalities, including an abnormal heart rhythm and various electrocardiographic (ECG) abnormalities. Fresh fruits and vegetables are good dietary sources of potassium. The body responds to the influx of dietary potassium, which raises serum potassium levels, with a shift of potassium from outside to inside cells, and an increase in potassium excretion by the kidney.