Spring 2007 Colloquium Series Physics Department University of Oregon 4:00pm Thursdays, 100 Willamette
... driving force behind many scientific and technological breakthroughs over the past century, including the invention of the laser and the realization of ultracold atoms. Creating and preserving optical phase coherence are one of the two major ingredients (the other being the control of matter) for th ...
... driving force behind many scientific and technological breakthroughs over the past century, including the invention of the laser and the realization of ultracold atoms. Creating and preserving optical phase coherence are one of the two major ingredients (the other being the control of matter) for th ...
Simple Harmonic Oscillator
... Particles are still in a superposition of two possible positions… …but position of each particle depends on the other: if particle 1 went through right slit, particle 2 went through left, and vice versa. ...
... Particles are still in a superposition of two possible positions… …but position of each particle depends on the other: if particle 1 went through right slit, particle 2 went through left, and vice versa. ...
Quantum Number, n. - Lyndhurst Schools
... where n is the principal quantum number (i.e., n = 1, 2, 3, … and nothing else). ...
... where n is the principal quantum number (i.e., n = 1, 2, 3, … and nothing else). ...
Document
... when Uo ~ 4 eV), tunnel current is very sensitive to variations in z as tip is scanned across surface. ...
... when Uo ~ 4 eV), tunnel current is very sensitive to variations in z as tip is scanned across surface. ...
Slide 1
... Used the idea of quanta to explain the photoelectric effect. • He proposed that light behaves as a stream of particles called photons • A photon’s energy must exceed a minimum threshold for electrons to be ejected. ...
... Used the idea of quanta to explain the photoelectric effect. • He proposed that light behaves as a stream of particles called photons • A photon’s energy must exceed a minimum threshold for electrons to be ejected. ...
collapses - Marc Madou
... knew the positions and motion of all the particles in the Universe, then we could calculate their behavior at any other time, in the past or the future.” In classical physics, particles and trajectories are real entities and it is assumed that the universe exists independently from the observer, tha ...
... knew the positions and motion of all the particles in the Universe, then we could calculate their behavior at any other time, in the past or the future.” In classical physics, particles and trajectories are real entities and it is assumed that the universe exists independently from the observer, tha ...
Quantum Mechanics Booklet
... will never be able to, understand. Scientists need to realise that there is another force working outside the world which has given it the regular laws of nature (Newton’s laws) as well as the strange laws of quantum mechanics. The reason that we cannot understand the workings of quantum physics is ...
... will never be able to, understand. Scientists need to realise that there is another force working outside the world which has given it the regular laws of nature (Newton’s laws) as well as the strange laws of quantum mechanics. The reason that we cannot understand the workings of quantum physics is ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI-600034 M.Sc. Part-A NOVEMBER 2015
... Convert the Cartesian coordinate (-1,1,-√2) into spherical polar coordinates. Derive time independent Schrodinger wave equation from time dependent equation. The force constant for H79Br is 392 Nm-1. Calculate the fundamental vibrational frequency and zero point energy of H79Br. Use the method of se ...
... Convert the Cartesian coordinate (-1,1,-√2) into spherical polar coordinates. Derive time independent Schrodinger wave equation from time dependent equation. The force constant for H79Br is 392 Nm-1. Calculate the fundamental vibrational frequency and zero point energy of H79Br. Use the method of se ...
Lecture 1
... 1923 Compton discovers the quantum (particle) nature of x rays, thus confirming photons as particles. 1924 de Broglie proposes that matter has wave properties. 1925 Pauli formulates the exclusion principle for electrons in an atom. Bothe and Geiger demonstrate that energy and mass are conserved in a ...
... 1923 Compton discovers the quantum (particle) nature of x rays, thus confirming photons as particles. 1924 de Broglie proposes that matter has wave properties. 1925 Pauli formulates the exclusion principle for electrons in an atom. Bothe and Geiger demonstrate that energy and mass are conserved in a ...
slides - University of Colorado Boulder
... “The simulations were the best part of class, they practically answer physics questions all by themselves. I would recommend continuing to develop these and add more. Without these I think I would have been lost in the course.” “I definitely not only enjoyed the simulations, but I'd go as far to say ...
... “The simulations were the best part of class, they practically answer physics questions all by themselves. I would recommend continuing to develop these and add more. Without these I think I would have been lost in the course.” “I definitely not only enjoyed the simulations, but I'd go as far to say ...
Nanoscience
... While an electron is moving, don't think of it as a particle that follows a particular path through space. A wave follows many paths simultaneously. Although an electron was used as an example here, the same could be said about other particles like protons, neutrons, or photons. It is even possible ...
... While an electron is moving, don't think of it as a particle that follows a particular path through space. A wave follows many paths simultaneously. Although an electron was used as an example here, the same could be said about other particles like protons, neutrons, or photons. It is even possible ...
quarks
... 1923 Compton discovers the quantum (particle) nature of x rays, thus confirming photons as particles. 1924 de Broglie proposes that matter has wave properties. 1925 Pauli formulates the exclusion principle for electrons in an atom. Bothe and Geiger demonstrate that energy and mass are conserved in a ...
... 1923 Compton discovers the quantum (particle) nature of x rays, thus confirming photons as particles. 1924 de Broglie proposes that matter has wave properties. 1925 Pauli formulates the exclusion principle for electrons in an atom. Bothe and Geiger demonstrate that energy and mass are conserved in a ...
What is a Force?
... This particle would be holding not only protons to protons but protons to neutrons and neutrons to neutrons He predicted the properties the new particle should have. The neutral pion (π0) was discovered in 1947 and it was thought to be totally responsible for the strong force. We now know this is no ...
... This particle would be holding not only protons to protons but protons to neutrons and neutrons to neutrons He predicted the properties the new particle should have. The neutral pion (π0) was discovered in 1947 and it was thought to be totally responsible for the strong force. We now know this is no ...
Planck-Einstein relation, Time Dep. Schrodinger Eq., Po
... ν and speed c by λ ν = c, and the wave vector k is given by k = 2π/λ . Combining these three relations with the Planck-Einstein relation for quantization of light, E = hν, immediately yields p = h̄k. Note also that for photons ω = ck where ω = 2πν is the angular frequency, i.e., there is a linear r ...
... ν and speed c by λ ν = c, and the wave vector k is given by k = 2π/λ . Combining these three relations with the Planck-Einstein relation for quantization of light, E = hν, immediately yields p = h̄k. Note also that for photons ω = ck where ω = 2πν is the angular frequency, i.e., there is a linear r ...
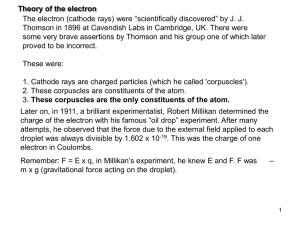
electron_theory
... Two types of experimental evidence which arose in the 1920s suggested an additional property of the electron. One was the closely spaced splitting of the hydrogen spectral lines, called fine structure. The other was the Stern-Gerlach experiment which showed in 1922 that a beam of silver atoms direct ...
... Two types of experimental evidence which arose in the 1920s suggested an additional property of the electron. One was the closely spaced splitting of the hydrogen spectral lines, called fine structure. The other was the Stern-Gerlach experiment which showed in 1922 that a beam of silver atoms direct ...
Physics 564 – Particle Physics
... • Course dedicated to Standard Model and its extensions, eg. Phys 565. ...
... • Course dedicated to Standard Model and its extensions, eg. Phys 565. ...