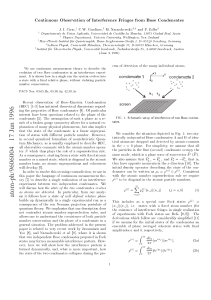
Summary of Important Ideas in Quantum Physics
... observable in day-to-day life because the intervals between the units are incredibly small. 2) At the atomic level, the behavior of particles is not classical; i.e., they cannot be described by Newtonian physics. Indeed, “particles” as such do not exist at the atomic level. The “position” of the ent ...
... observable in day-to-day life because the intervals between the units are incredibly small. 2) At the atomic level, the behavior of particles is not classical; i.e., they cannot be described by Newtonian physics. Indeed, “particles” as such do not exist at the atomic level. The “position” of the ent ...
Chapter_5
... • Not only does light behave as a wave, it also behaves as a particle. • Einstein’s explanation of the photoelectric effect helped display this quality. • The effect says that electrons are ejected from the surface of a polished metal plate when it is struck by light. ...
... • Not only does light behave as a wave, it also behaves as a particle. • Einstein’s explanation of the photoelectric effect helped display this quality. • The effect says that electrons are ejected from the surface of a polished metal plate when it is struck by light. ...
(Electromagnetic Wave).
... Before the 17th Century, most people believed that light traveled instantaneously. Galileo first hypothesized that light has a finite speed. Ole Roemer made 70 careful measurements of the lunar eclipse of Io (a moon of Jupiter) from ...
... Before the 17th Century, most people believed that light traveled instantaneously. Galileo first hypothesized that light has a finite speed. Ole Roemer made 70 careful measurements of the lunar eclipse of Io (a moon of Jupiter) from ...
Waves - TeacherWeb
... displacement of a medium by two or more waves is the algebraic sum of the displacement of the individual waves. Waves can either have constructive interference or destructive interference. When waves cancel and result in no displacement, this is called a node. When waves build and result in increase ...
... displacement of a medium by two or more waves is the algebraic sum of the displacement of the individual waves. Waves can either have constructive interference or destructive interference. When waves cancel and result in no displacement, this is called a node. When waves build and result in increase ...
Supercomputing in High Energy Physics
... Particle Physics “Everything in the universe seems to be made of simple, small objects which like to stick together” • Modern realization of this: The Standard Model – A quantum field theory in which point-like, spin-1/2 fermions interact through the exchange of spin-1 vector ...
... Particle Physics “Everything in the universe seems to be made of simple, small objects which like to stick together” • Modern realization of this: The Standard Model – A quantum field theory in which point-like, spin-1/2 fermions interact through the exchange of spin-1 vector ...
wave
... Albert Michelson is known for making an definitive measurement of the speed of light in the late 1800’s to early 1900’s, using a rotating mirror apparatus between 2 California mountains 22 miles apart. His value: 299,796 km/s. Accepted value today: 299,792 km/s He was the first American to win the N ...
... Albert Michelson is known for making an definitive measurement of the speed of light in the late 1800’s to early 1900’s, using a rotating mirror apparatus between 2 California mountains 22 miles apart. His value: 299,796 km/s. Accepted value today: 299,792 km/s He was the first American to win the N ...
uncertainty, atom
... What it means: You cannot know position and momentum both very precisely at the same time If you measure momentum, you disturb the position, so you no longer know the position accurately -- and vice versa This disturbance is random, indeterminate (unlike letting a little air out when you measure ...
... What it means: You cannot know position and momentum both very precisely at the same time If you measure momentum, you disturb the position, so you no longer know the position accurately -- and vice versa This disturbance is random, indeterminate (unlike letting a little air out when you measure ...
Blackbody Radiation and Planck`s Hypothesis of Quantized Energy
... This is even true if we have a particle beam so weak that only one particle is present at a time – we still see the diffraction pattern produced by constructive and destructive interference. Also, as the diffraction pattern builds, we cannot predict where any particular particle will land, although ...
... This is even true if we have a particle beam so weak that only one particle is present at a time – we still see the diffraction pattern produced by constructive and destructive interference. Also, as the diffraction pattern builds, we cannot predict where any particular particle will land, although ...
quantum number
... What this means is that it is not possible to assign a definite position for a particle in a system. All that can be given is the probability of finding the particle at a particular location. This is why, for example, we describe the electrons in an atom as a “cloud” of charge surrounding the ...
... What this means is that it is not possible to assign a definite position for a particle in a system. All that can be given is the probability of finding the particle at a particular location. This is why, for example, we describe the electrons in an atom as a “cloud” of charge surrounding the ...
Chapter 7 Quantum Theory of the Atom
... by absorbing or emitting a photon Energy of a photon is the difference in energy between the energy levels Emission of light during a transition gives the line spectrum of the element results from an e– moving from a higher energy level to a lower energy level Energy of an emitted photon ...
... by absorbing or emitting a photon Energy of a photon is the difference in energy between the energy levels Emission of light during a transition gives the line spectrum of the element results from an e– moving from a higher energy level to a lower energy level Energy of an emitted photon ...
Chapter 4 - Tolland High School
... • The Bohr model was more accurate than previous models but was only completely accurate for Hydrogen, other elements did not behave exactly as Bohr predicted • The Quantum model was later developed based on work of many scientists including Schrodinger, Heisenberg, & Einstein ...
... • The Bohr model was more accurate than previous models but was only completely accurate for Hydrogen, other elements did not behave exactly as Bohr predicted • The Quantum model was later developed based on work of many scientists including Schrodinger, Heisenberg, & Einstein ...
Physics 1020 Ch 10-12 Practice Exam (2).
... b. any electron present in an atom can have the same quantum state, since all electrons in an atom have the same mass and charge. c. there can be infinitely amount of electrons occupying an orbital as long as enough energy is provided. d. no two electrons can occupy the same quantum state. 11. The A ...
... b. any electron present in an atom can have the same quantum state, since all electrons in an atom have the same mass and charge. c. there can be infinitely amount of electrons occupying an orbital as long as enough energy is provided. d. no two electrons can occupy the same quantum state. 11. The A ...
Chapter 01
... Any algorithmic process can be simulated efficiently using a Turing machine. (People have tried to find a counter example, for instance, primality) • Randomized algorithms (an ad-hoc version of strong Church–Turing thesis) Any algorithmic process can be simulated efficiently using a probabilistic TM ...
... Any algorithmic process can be simulated efficiently using a Turing machine. (People have tried to find a counter example, for instance, primality) • Randomized algorithms (an ad-hoc version of strong Church–Turing thesis) Any algorithmic process can be simulated efficiently using a probabilistic TM ...
PARTICLE IN AN INFINITE POTENTIAL WELL
... This process may be performed for any other observable. For example, the average momentum or the expectation value of the momentum of a particle in the n-th state of the box. The only difference in the procedure to determine the expectation value of position is to replace the position operator with ...
... This process may be performed for any other observable. For example, the average momentum or the expectation value of the momentum of a particle in the n-th state of the box. The only difference in the procedure to determine the expectation value of position is to replace the position operator with ...