Atomic Structure
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
AtomicStructure
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
... Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons Electrons have so little mass that atoms must contain other particles that account for most of the mass ...
Entanglement of Identical Particles
... particle, constantly accelerating and not reaching the velocity of light because the acceleration is radial. One origin of the Quantum Physics is the Planck Distribution Law of the electromagnetic ...
... particle, constantly accelerating and not reaching the velocity of light because the acceleration is radial. One origin of the Quantum Physics is the Planck Distribution Law of the electromagnetic ...
Modern physics
... spacing on the grating. Electron wavelength is hundreds of times shorter that the wavelengths of visible light. ...
... spacing on the grating. Electron wavelength is hundreds of times shorter that the wavelengths of visible light. ...
The Heisenberg Uncertainty Principle
... Principle The Heisenberg uncertainty principle states that it is impossible to know both the momentum and the position of a particle at the same time. This limitation is critical when dealing with small particles such as electrons. But it does not matter for ordinary-sized objects such as cars o ...
... Principle The Heisenberg uncertainty principle states that it is impossible to know both the momentum and the position of a particle at the same time. This limitation is critical when dealing with small particles such as electrons. But it does not matter for ordinary-sized objects such as cars o ...
How have advances in particle accelerator technology helped the
... larger mass is collides, the products will have greater mass due to the additional mass and energy input. At SLAC, a slightly different method is used; electrons and their anti particles, positrons are both accelerated. At the end of the accelerator they are split into a storage ring, the electrons ...
... larger mass is collides, the products will have greater mass due to the additional mass and energy input. At SLAC, a slightly different method is used; electrons and their anti particles, positrons are both accelerated. At the end of the accelerator they are split into a storage ring, the electrons ...
PPT
... = detecting light that has been reflected off the object's surface light = electromagnetic wave; “visible light”= those electromagnetic waves that our eyes can detect “wavelength” of e.m. wave (distance between two successive crests) determines “color” of light wave hardly influenced by object if ...
... = detecting light that has been reflected off the object's surface light = electromagnetic wave; “visible light”= those electromagnetic waves that our eyes can detect “wavelength” of e.m. wave (distance between two successive crests) determines “color” of light wave hardly influenced by object if ...
Brief history of the atom
... However, no one could determine the exact cause of the spectral lines. According to Rutherford’s model, electrons that gave off continuous energy would spiral into the nucleus of the atom. Another model of the atom had to be made. ...
... However, no one could determine the exact cause of the spectral lines. According to Rutherford’s model, electrons that gave off continuous energy would spiral into the nucleus of the atom. Another model of the atom had to be made. ...
Orbital
... The brilliant red colors seen in fireworks are due to the emission of light with wavelengths around 650 nm when strontium salts such as Sr(NO3)2 and SrCO3 are heated. (This can be easily demonstrated in the lab by dissolving ones of these salts in methanol that contains a little water and igniting t ...
... The brilliant red colors seen in fireworks are due to the emission of light with wavelengths around 650 nm when strontium salts such as Sr(NO3)2 and SrCO3 are heated. (This can be easily demonstrated in the lab by dissolving ones of these salts in methanol that contains a little water and igniting t ...
6.1.2. Number Representation: States
... Consider a set of complete, orthonormal 1-particle (1-P) basis. For the sake of clarity, we shall assume the quantum numbers to be discrete. (Results for the continuous case can be obtained by some limiting procedure). To begin, we arrange the 1-P states by some rule into a unique sequence 0,1,2 ...
... Consider a set of complete, orthonormal 1-particle (1-P) basis. For the sake of clarity, we shall assume the quantum numbers to be discrete. (Results for the continuous case can be obtained by some limiting procedure). To begin, we arrange the 1-P states by some rule into a unique sequence 0,1,2 ...
Text Book: Fundamentals of Physics Authors: Halliday, Resnick
... increases to a maximum and then decreases gradually in the layer wavelengths. (ii) A line spectrum consisting of groups or series of two or three peaks of high-intensity radiation superimposed on the continuous spectrum. The series are denoted by the letter K, L, M etc. ...
... increases to a maximum and then decreases gradually in the layer wavelengths. (ii) A line spectrum consisting of groups or series of two or three peaks of high-intensity radiation superimposed on the continuous spectrum. The series are denoted by the letter K, L, M etc. ...
Chapter 4-2 The Quantum Model of the Atom
... as a wave and as a particle. Louis de Broglie asked if electrons could behave the same way as light. de Broglie suggested that electrons be considered waves confined to the space around the atomic nucleus. It turns out that electrons do have wavelike properties. ...
... as a wave and as a particle. Louis de Broglie asked if electrons could behave the same way as light. de Broglie suggested that electrons be considered waves confined to the space around the atomic nucleus. It turns out that electrons do have wavelike properties. ...
Nobel Lecture: One hundred years of light quanta*
... 共Einstein, 1905兲. The light itself, he assumed, consists of localized energy packets and each possesses one quantum of energy. When light strikes the metal, each packet is absorbed by a single electron. That electron then flies off with a unique energy, an energy which is just the packet energy h m ...
... 共Einstein, 1905兲. The light itself, he assumed, consists of localized energy packets and each possesses one quantum of energy. When light strikes the metal, each packet is absorbed by a single electron. That electron then flies off with a unique energy, an energy which is just the packet energy h m ...
No Slide Title
... (gradV)t t 1 t (1) 2m We can determine the first term on the right side of eq(1) By a Taylor expansion of the velocity ...
... (gradV)t t 1 t (1) 2m We can determine the first term on the right side of eq(1) By a Taylor expansion of the velocity ...
4.2 Notes - Seymour ISD
... Electrons as Waves • French scientist Louis de Broglie suggested that electrons be considered waves confined to the space around an atomic nucleus. • It followed that the electron waves could exist only at specific frequencies. • According to the relationship E = hν, these frequencies corresponded t ...
... Electrons as Waves • French scientist Louis de Broglie suggested that electrons be considered waves confined to the space around an atomic nucleus. • It followed that the electron waves could exist only at specific frequencies. • According to the relationship E = hν, these frequencies corresponded t ...
Columbia Science Honors Program - TWiki
... particles to very high energies, near the speed of light, c. At these speeds, Newtonian mechanics is superseded by special relativity. Elementary particle physics describes objects that are both very small and ...
... particles to very high energies, near the speed of light, c. At these speeds, Newtonian mechanics is superseded by special relativity. Elementary particle physics describes objects that are both very small and ...
Energy and Matter - Hicksville Public Schools
... The quantum mechanical model (cloud or Schrödinger model) of the atom explains the properties of atoms by treating the electron as a wave and by including the idea of quantized energies. Electrons occupy atomic orbitals. An orbital is a region in the space around the atom’s nucleus where an electron ...
... The quantum mechanical model (cloud or Schrödinger model) of the atom explains the properties of atoms by treating the electron as a wave and by including the idea of quantized energies. Electrons occupy atomic orbitals. An orbital is a region in the space around the atom’s nucleus where an electron ...
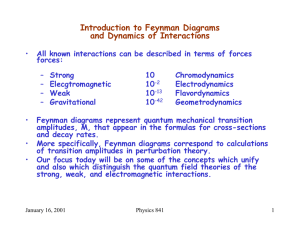
Introduction to Feynman Diagrams and Dynamics of Interactions
... perturbation theory in nonrelativistic quantum mechanics, we have second order perturbation theory in quantum field theories. ...
... perturbation theory in nonrelativistic quantum mechanics, we have second order perturbation theory in quantum field theories. ...