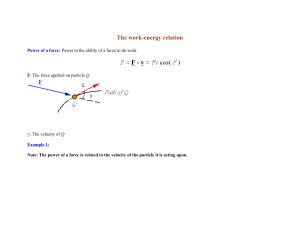
The work-energy relation
... kinetic energy of the particle. In other words, the kinetic energy of a particle is changed by an amount equal to the work which is done on the particle by the resultant force. ...
... kinetic energy of the particle. In other words, the kinetic energy of a particle is changed by an amount equal to the work which is done on the particle by the resultant force. ...
MS PowerPoint template
... the forces due the artificial potential field to the user through the haptic device for achieving better results in manipulation and control. ...
... the forces due the artificial potential field to the user through the haptic device for achieving better results in manipulation and control. ...
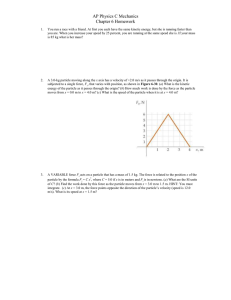
Chapter 6 Homework
... You run a race with a friend. At first you each have the same kinetic energy, but she is running faster than you are. When you increase your speed by 25 percent, you are running at the same speed she is. If your mass is 85 kg what is her mass? ...
... You run a race with a friend. At first you each have the same kinetic energy, but she is running faster than you are. When you increase your speed by 25 percent, you are running at the same speed she is. If your mass is 85 kg what is her mass? ...
Adobe Acrobat file () - Wayne State University Physics and
... In an analysis relating Bohr's theory to the de Broglie wavelength of electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference of its orbit becomes 9 times greater. This occurs because (a) there are 3 times as many wavelengths in the new orbit, (b) there are 3 tim ...
... In an analysis relating Bohr's theory to the de Broglie wavelength of electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference of its orbit becomes 9 times greater. This occurs because (a) there are 3 times as many wavelengths in the new orbit, (b) there are 3 tim ...
THE HISTORY OF THE ATOM Table of Contents Black Boxes
... Rutherford and the Nucleus In 1908, Ernest Rutherford performed the Gold Foil Experiment. In it, he shot alpha particles (very small, very dense, very fast particles) at a thin layer of gold foil. He expected all of the alpha particles to go straight through It would be like if you were shooting ...
... Rutherford and the Nucleus In 1908, Ernest Rutherford performed the Gold Foil Experiment. In it, he shot alpha particles (very small, very dense, very fast particles) at a thin layer of gold foil. He expected all of the alpha particles to go straight through It would be like if you were shooting ...
Elementary Particles Thornton and Rex, Ch. 13
... mass 135 MeV/c2. It is its own antiparticle. It lives about 8.4x10-17 seconds before decaying into two photons. p ...
... mass 135 MeV/c2. It is its own antiparticle. It lives about 8.4x10-17 seconds before decaying into two photons. p ...
PHOTONS AND PHOTON STATISTICS
... wave properties like interference fringes. It was expected that such fringes fade out if the intensity of the incident light becomes smaller and smaller so that the probability of having more than a single photon in the spectrometer becomes negligible. Interference experiments at very low intensity ...
... wave properties like interference fringes. It was expected that such fringes fade out if the intensity of the incident light becomes smaller and smaller so that the probability of having more than a single photon in the spectrometer becomes negligible. Interference experiments at very low intensity ...
Quantum Computers
... other polarization (say V). • If light polarized 45° to H and V arrives, half of it is reflected and half transmitted. • If a single photon at 45° arrives, it will be reflected or transmitted with 50/50 probability. We describe such a photon as a superposition of H and V: (|H>+|V>)/sqrt(2). ...
... other polarization (say V). • If light polarized 45° to H and V arrives, half of it is reflected and half transmitted. • If a single photon at 45° arrives, it will be reflected or transmitted with 50/50 probability. We describe such a photon as a superposition of H and V: (|H>+|V>)/sqrt(2). ...
Quantum Mechanics and Atomic Structure
... ---- OXTOBY OVERHEAD ------- DEMO WITH MAGNETS ---Here’s the idea: Apply a high voltage between metal electrodes in a nearly evacuated tube. Negatively charged particles are emitted from the negative electrode (cathode). These “cathode rays” are accelerated toward and through the positive electrode ...
... ---- OXTOBY OVERHEAD ------- DEMO WITH MAGNETS ---Here’s the idea: Apply a high voltage between metal electrodes in a nearly evacuated tube. Negatively charged particles are emitted from the negative electrode (cathode). These “cathode rays” are accelerated toward and through the positive electrode ...
Do your homework on a separate piece of paper, or
... 20. What is meant by the term “wave-particle duality” and to what is it applied? Matter and energy can both act like waves and act like particles. 21. What is a matter wave? It is the wave part of the duality manifest in matter. = h/p. 22. State the de Broglie hypothesis, and then write its mathem ...
... 20. What is meant by the term “wave-particle duality” and to what is it applied? Matter and energy can both act like waves and act like particles. 21. What is a matter wave? It is the wave part of the duality manifest in matter. = h/p. 22. State the de Broglie hypothesis, and then write its mathem ...
EXPERIMENT 17 To Determine Avogadro`s Number by
... viscosity of the fluid in which it was suspended and the temperature of the fluid. Many observers noted that these particles appeared to behave in the same way as the molecules of an ideal gas should behave according to the Kinetic Theory. The explanation of Brownian Motion was first given by Einste ...
... viscosity of the fluid in which it was suspended and the temperature of the fluid. Many observers noted that these particles appeared to behave in the same way as the molecules of an ideal gas should behave according to the Kinetic Theory. The explanation of Brownian Motion was first given by Einste ...
Part 3: Quantum numbers and orbitals
... Review: in Bohr’s atomic model, electrons orbited the nucleus as shown below. To mathematically describe the orbit of an electron, Bohr used one quantum number, n = 1, 2, 3 ……which designated 2 things: ...
... Review: in Bohr’s atomic model, electrons orbited the nucleus as shown below. To mathematically describe the orbit of an electron, Bohr used one quantum number, n = 1, 2, 3 ……which designated 2 things: ...
Chapter 6
... •Louis de Broglie posited that if light can have material properties, matter should exhibit wave properties. •He demonstrated that the relationship between mass and wavelength was The Uncertainty Principle •Heisenberg showed that the more precisely the momentum of a particle is known, the less preci ...
... •Louis de Broglie posited that if light can have material properties, matter should exhibit wave properties. •He demonstrated that the relationship between mass and wavelength was The Uncertainty Principle •Heisenberg showed that the more precisely the momentum of a particle is known, the less preci ...