Swimming in a sea of light: the adventure of photon hydrodynamics
... So far we have seen that: Bose-condensed bosonic atoms below TBEC forget their corpuscular nature and behave as a macroscopic coherent matter wave ...
... So far we have seen that: Bose-condensed bosonic atoms below TBEC forget their corpuscular nature and behave as a macroscopic coherent matter wave ...
L01_5342_Sp02
... • A device of the student's choice may be used for one of the projects (by permission) • Format and content will be discussed when the project is assigned and will be included in the grade. L1 January 15 ...
... • A device of the student's choice may be used for one of the projects (by permission) • Format and content will be discussed when the project is assigned and will be included in the grade. L1 January 15 ...
Does Quantum Mechanics Make Sense?
... Quantum – know p exactly, x completely uncertain. Equal probability of finding particle anywhere. What about Einstein’s photons that are particles and electrons that are particles, but they both have momenta that are delocalized probability waves? Waves of different wavelengths can be ...
... Quantum – know p exactly, x completely uncertain. Equal probability of finding particle anywhere. What about Einstein’s photons that are particles and electrons that are particles, but they both have momenta that are delocalized probability waves? Waves of different wavelengths can be ...
Lecture 24 (7.1-7.2)
... – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained the energy profiles by assuming that the energy of an object can be changed only in discrete amounts (quanta) → ...
... – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained the energy profiles by assuming that the energy of an object can be changed only in discrete amounts (quanta) → ...
Khatua, Bansal, and Shahar Reply: The preceding
... Khatua, Bansal, and Shahar Reply: The preceding Comment [1] on our Letter [2] does not discuss any technical or mathematical aspects of the experiment or analysis but remarks on interpretational issues. These remarks are in turn based on the critique of Feynman’s thought experiment itself [3]: “a th ...
... Khatua, Bansal, and Shahar Reply: The preceding Comment [1] on our Letter [2] does not discuss any technical or mathematical aspects of the experiment or analysis but remarks on interpretational issues. These remarks are in turn based on the critique of Feynman’s thought experiment itself [3]: “a th ...
Read Notes #1 - Faculty Website Listing
... has changed. Classical Physics is DETERMINISTIC. In Classical physics it is possible at least theoretically for one to specify exactly both the position and velocity of a particle (i.e. its trajectory). This is what you did in homework problems in Introductory Physics. The Heisenberg uncertainty pri ...
... has changed. Classical Physics is DETERMINISTIC. In Classical physics it is possible at least theoretically for one to specify exactly both the position and velocity of a particle (i.e. its trajectory). This is what you did in homework problems in Introductory Physics. The Heisenberg uncertainty pri ...
Document
... • Compton showed Dp = hkinitial - hkfinal, so an photon (wave) is particle-like • DeBroglie hypothesized a particle could be wave-like, l = h/p • Davisson and Germer demonstrated wave-like interference phenomena for electrons to complete the duality model L1 January 18 ...
... • Compton showed Dp = hkinitial - hkfinal, so an photon (wave) is particle-like • DeBroglie hypothesized a particle could be wave-like, l = h/p • Davisson and Germer demonstrated wave-like interference phenomena for electrons to complete the duality model L1 January 18 ...
EE 5342 Lecture
... • Compton showed Dp = hkinitial - hkfinal, so an photon (wave) is particle-like • DeBroglie hypothesized a particle could be wave-like, l = h/p • Davisson and Germer demonstrated wave-like interference phenomena for electrons to complete the duality model L1 January 20 ...
... • Compton showed Dp = hkinitial - hkfinal, so an photon (wave) is particle-like • DeBroglie hypothesized a particle could be wave-like, l = h/p • Davisson and Germer demonstrated wave-like interference phenomena for electrons to complete the duality model L1 January 20 ...
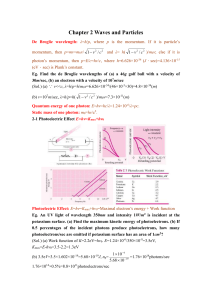
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
... (eV.sec) is Plank’s constant. Eg. Find the de Broglie wavelengths of (a) a 46g golf ball with a velocity of 30m/sec, (b) an electron with a velocity of 107m/sec (Sol.) (a) ∵ v<
... (eV.sec) is Plank’s constant. Eg. Find the de Broglie wavelengths of (a) a 46g golf ball with a velocity of 30m/sec, (b) an electron with a velocity of 107m/sec (Sol.) (a) ∵ v<
PH5015 - Applications of Quantum Physics
... evaluate the opportunities and limitations offered by atoms, ions, and photons in quantum physics experiments, and in particular be able to judge the applicability of various systems for experiments in this field. They should be able to use their understanding of the links between the quantum and cl ...
... evaluate the opportunities and limitations offered by atoms, ions, and photons in quantum physics experiments, and in particular be able to judge the applicability of various systems for experiments in this field. They should be able to use their understanding of the links between the quantum and cl ...
科目名稱:普通化學 期中考(I) 日期:99年10月18日 學號 姓名 I. 名詞
... 1. Calculate the wavelength (in nm) of a photon emitted by a hydrogen atom when its electron drops from the n = 5 state to the n = 3 state. (5%) 2. A cube made of platinum (Pt) has an edge length of 1.0 cm. (a) Calculate the number of Pt atoms in the cube, (b) Atoms are spherical in shape. Therefore ...
... 1. Calculate the wavelength (in nm) of a photon emitted by a hydrogen atom when its electron drops from the n = 5 state to the n = 3 state. (5%) 2. A cube made of platinum (Pt) has an edge length of 1.0 cm. (a) Calculate the number of Pt atoms in the cube, (b) Atoms are spherical in shape. Therefore ...










![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/003926344_1-b779c05b753c6dc3972377c21f9bdcd3-300x300.png)