Principles of Chemistry: A Molecular Approach
... All atoms of a g given element have the same mass and other properties that distinguish them from atoms of other elements. Atoms combine in simple, whole-number ratios to form molecules of compounds. In a chemical reaction, atoms of one element cannot change into atoms of another element. They sim ...
... All atoms of a g given element have the same mass and other properties that distinguish them from atoms of other elements. Atoms combine in simple, whole-number ratios to form molecules of compounds. In a chemical reaction, atoms of one element cannot change into atoms of another element. They sim ...
Transitions between highly excited states of an atom when a neutral
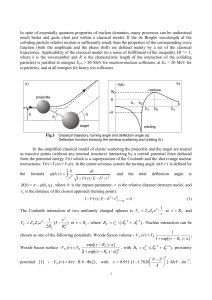
... collisions of the neutrals with the core. This fact was established for the system H(n) He(ls2)as a result of the comparison with calculation^^^^ based on the competing mechanism of elastic scattering of a Rydberg electron by a neutral p a r t i ~ l eThe . ~ latter mechanism leads only to establishm ...
... collisions of the neutrals with the core. This fact was established for the system H(n) He(ls2)as a result of the comparison with calculation^^^^ based on the competing mechanism of elastic scattering of a Rydberg electron by a neutral p a r t i ~ l eThe . ~ latter mechanism leads only to establishm ...
Teleportation of an atomic state between two cavities using nonlocal
... strong coupling to microwaves and their very long radiative decay times, circular levels are ideally suited [11]for preparing and detecting long-lived correlations between atom and field states. Atoms in circular Rydberg states, which can be prepared on each beam at a given time with a well-defined ...
... strong coupling to microwaves and their very long radiative decay times, circular levels are ideally suited [11]for preparing and detecting long-lived correlations between atom and field states. Atoms in circular Rydberg states, which can be prepared on each beam at a given time with a well-defined ...
Quantum statistics: Is there an effective fermion repulsion or boson
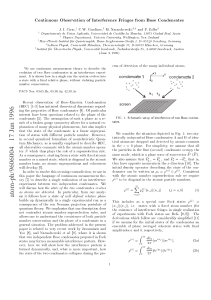
... This concept has been with physics since the early days of quantum mechanics. Nevertheless, it is important to examine the usefulness of this heuristic interpretation of the mathematics. As Layzer has pointed out,1 no such interpretation can carry the whole weight of the rigorous mathematical formul ...
... This concept has been with physics since the early days of quantum mechanics. Nevertheless, it is important to examine the usefulness of this heuristic interpretation of the mathematics. As Layzer has pointed out,1 no such interpretation can carry the whole weight of the rigorous mathematical formul ...
Chapter 2 The Ideal Gas
... the interparticle forces are negligible. It is a reasonably accurate model of a real gas at low density. Although it will be assumed that the interparticle forces are weak enough to be neglected, one should not picture the ideal gas as a system of particles with no interactions at all. Such a system ...
... the interparticle forces are negligible. It is a reasonably accurate model of a real gas at low density. Although it will be assumed that the interparticle forces are weak enough to be neglected, one should not picture the ideal gas as a system of particles with no interactions at all. Such a system ...
Geiger–Marsden experiment
The Geiger–Marsden experiment(s) (also called the Rutherford gold foil experiment) were a landmark series of experiments by which scientists discovered that every atom contains a nucleus where its positive charge and most of its mass are concentrated. They deduced this by measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The experiments were performed between 1908 and 1913 by Hans Geiger and Ernest Marsden under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester.