terpconnect.umd.edu
... Rayleigh limit: the equilibrium state at which further addition of charge will cause the drop to become unstable and break ...
... Rayleigh limit: the equilibrium state at which further addition of charge will cause the drop to become unstable and break ...
ionization 12.3.1
... Describes the process whereby new ionized species are formed when gaseous molecules interact with ions. The process may involve transfer of an electron, a proton or other charged species between the reactants. When a positive ion results from chemical ionization the term may be used without qualific ...
... Describes the process whereby new ionized species are formed when gaseous molecules interact with ions. The process may involve transfer of an electron, a proton or other charged species between the reactants. When a positive ion results from chemical ionization the term may be used without qualific ...
Fate and Transport of Air Pollutants from CAFOs
... deposition is measured with a bucket collector and a rain gauge. The rain gauge is placed at the receptor site and provides an accurate measure of precipitation rate, I. The wet bucket collector is open only during the precipitation event, and its contents are analyzed for pollutant concentration, C ...
... deposition is measured with a bucket collector and a rain gauge. The rain gauge is placed at the receptor site and provides an accurate measure of precipitation rate, I. The wet bucket collector is open only during the precipitation event, and its contents are analyzed for pollutant concentration, C ...
A single parameter representation of hygroscopic
... Abstract. We present a method to describe the relationship between particle dry diameter and cloud condensation nuclei (CCN) activity using a single hygroscopicity parameter κ. Values of the hygroscopicity parameter are between 0.5 and 1.4 for highly-CCN-active salts such as sodium chloride, between ...
... Abstract. We present a method to describe the relationship between particle dry diameter and cloud condensation nuclei (CCN) activity using a single hygroscopicity parameter κ. Values of the hygroscopicity parameter are between 0.5 and 1.4 for highly-CCN-active salts such as sodium chloride, between ...
In Situ Raman Spectroscopic Study of Gypsum (CaSO4·2H2O) and
... studied the stepwise dehydration of levitated epsomite (MgSO4·7H2O) and gypsum (CaSO4·2H2O) crystals induced by heating from the carbon dioxide laser. These experiments have important implications for the understanding of our solar system. The OMEGA/Mars Express hyperspectral imager identified hydrat ...
... studied the stepwise dehydration of levitated epsomite (MgSO4·7H2O) and gypsum (CaSO4·2H2O) crystals induced by heating from the carbon dioxide laser. These experiments have important implications for the understanding of our solar system. The OMEGA/Mars Express hyperspectral imager identified hydrat ...
Effect of Sulfuric Acid Manufacturing Process on
... Approaches to identify the root cause of these defects; (1) Failure analysis of the particles, (2) Comparison of defects from various components of the production recipe to conclude the defect source, (3) Comparison of defects from cleans process recipes with different SPM dispense times, and (4) An ...
... Approaches to identify the root cause of these defects; (1) Failure analysis of the particles, (2) Comparison of defects from various components of the production recipe to conclude the defect source, (3) Comparison of defects from cleans process recipes with different SPM dispense times, and (4) An ...
FeCo magnetic nanoneedles obtained by Co-coating
... 10% of Co can be deposited onto the surface of the haematite particles (figure 2(b)), while higher amounts, like 20% and 30% of Co, resulted in a different phase segregated from the haematite particles, as can be observed in figure 2(c). This is clearly illustrated by x-ray diffraction in figure 3, ...
... 10% of Co can be deposited onto the surface of the haematite particles (figure 2(b)), while higher amounts, like 20% and 30% of Co, resulted in a different phase segregated from the haematite particles, as can be observed in figure 2(c). This is clearly illustrated by x-ray diffraction in figure 3, ...
Compulsory textbook Recommended textbooks Topics of the first
... how much is present? – percentage or mass of the analyte in the sample Separation techniques (chromatographies) different components may interfere one with another ...
... how much is present? – percentage or mass of the analyte in the sample Separation techniques (chromatographies) different components may interfere one with another ...
Cloud droplet activation and surface tension of mixtures of slightly
... major role for the activation of particles consisting of slightly soluble organic compounds (Bilde and Svenningsson, 2004; Hori et al., 2003; Broekhuizen et al., 2004). In the atmosphere particles are most likely to be mixtures of organic and inorganic components. Even if a purely organic particle e ...
... major role for the activation of particles consisting of slightly soluble organic compounds (Bilde and Svenningsson, 2004; Hori et al., 2003; Broekhuizen et al., 2004). In the atmosphere particles are most likely to be mixtures of organic and inorganic components. Even if a purely organic particle e ...
File
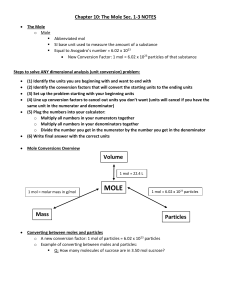
... Assume you have 100 g of the compound. Change “%” to “g” Convert grams to moles for each element Divide each mole amount by the smallest mole amount, these numbers are the coefficients for the E.F. If the numbers from step 4 are not all whole numbers, multiply the step 4 numbers by a whole number so ...
... Assume you have 100 g of the compound. Change “%” to “g” Convert grams to moles for each element Divide each mole amount by the smallest mole amount, these numbers are the coefficients for the E.F. If the numbers from step 4 are not all whole numbers, multiply the step 4 numbers by a whole number so ...
Limiting reactant - Dr. Gregory Chemistry
... relationships between the amounts of reactants used and amounts of products formed in a chemical reaction. It is based on the law of conservation of mass. ...
... relationships between the amounts of reactants used and amounts of products formed in a chemical reaction. It is based on the law of conservation of mass. ...
SAFETY DATA SHEET Fast Dry Enamel Aerosol 100 ml
... Dangerous Preparations Directive 1999/45/EC. Aerosol directive 75/324/EEC as amended by 94/01/EEC. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing ...
... Dangerous Preparations Directive 1999/45/EC. Aerosol directive 75/324/EEC as amended by 94/01/EEC. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing ...
Size-Resolved Kinetic Measurements of Aluminum Nanoparticle
... with a fused silica plano-convex lens of focal length 100 mm to a spot diameter of the order of ∼1 mm at the aluminum pellet surface positioned near the focal point of the lens. The focused laser beam has an energy density of approximately 1 × 1010 W/cm2. This generates a local microplasma at the su ...
... with a fused silica plano-convex lens of focal length 100 mm to a spot diameter of the order of ∼1 mm at the aluminum pellet surface positioned near the focal point of the lens. The focused laser beam has an energy density of approximately 1 × 1010 W/cm2. This generates a local microplasma at the su ...
Volatility of Organic Aerosol: Evaporation of Ammonium Sulfate
... multicomponent mixtures. Activity models are often developed based on water equilibrium, rather than the equilibrium of the organic solute − largely due to the fact that experimental data on organic activities are extremely scarce. Also activity models for mixtures of inorganic and organic solutes h ...
... multicomponent mixtures. Activity models are often developed based on water equilibrium, rather than the equilibrium of the organic solute − largely due to the fact that experimental data on organic activities are extremely scarce. Also activity models for mixtures of inorganic and organic solutes h ...
Investigating Freezing Point Depression and Cirrus Cloud
... the formation of cirrus clouds. To understand the effect cirrus clouds have on the Earth’s radiation budget, it is important to understand the conditions at which they form. Therefore, it is crucial to determine how dissolved solutes affect the freezing and melting temperatures of micrometer-sized aer ...
... the formation of cirrus clouds. To understand the effect cirrus clouds have on the Earth’s radiation budget, it is important to understand the conditions at which they form. Therefore, it is crucial to determine how dissolved solutes affect the freezing and melting temperatures of micrometer-sized aer ...
Monodisperse FePt Nanoparticles and Ferromagnetic FePt
... 3:2 molar ratio of Fe(CO)5 to Pt(acac)2 gave Fe48Pt52 particles, a 2 :1 molar ratio yielded Fe52Pt48, and a 4 :1 molar ratio produced Fe70Pt30 (22). The FePt particle size can be tuned from 3 to 10 nm by first growing 3-nm monodisperse seed particles in situ and then adding more reagents to enlarge ...
... 3:2 molar ratio of Fe(CO)5 to Pt(acac)2 gave Fe48Pt52 particles, a 2 :1 molar ratio yielded Fe52Pt48, and a 4 :1 molar ratio produced Fe70Pt30 (22). The FePt particle size can be tuned from 3 to 10 nm by first growing 3-nm monodisperse seed particles in situ and then adding more reagents to enlarge ...
Figure 4 - University of Wisconsin–Madison
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
Introduction to Organic Mass Spectrometry
... Multiplexing: all m/z ratios analyzed simultaneously (cf FTIR or PDA) Time-of-flight ...
... Multiplexing: all m/z ratios analyzed simultaneously (cf FTIR or PDA) Time-of-flight ...
Ministry Strand: Quantities in Chemical Reactions Teacher
... - To begin lesson, instructor will show 4 diagrams on SMART Board: ...
... - To begin lesson, instructor will show 4 diagrams on SMART Board: ...
Jon Abbatt - Earth, Atmospheric, and Planetary Physics
... ClO + O2, OH + CH4 H2O + CH3) or it is photochemical (e.g. HNO3 OH + NO2, CF2Cl2 + hv Cl + CF2Cl). Molecules that have all their electrons paired up do not react together at atmospherically significant rates in the gas phase. Particles can promote reactions that do not proceed in the gas-phase ...
... ClO + O2, OH + CH4 H2O + CH3) or it is photochemical (e.g. HNO3 OH + NO2, CF2Cl2 + hv Cl + CF2Cl). Molecules that have all their electrons paired up do not react together at atmospherically significant rates in the gas phase. Particles can promote reactions that do not proceed in the gas-phase ...
Molar Mass - Science With Horne
... The mole (abbreviated mol) is the base unit for measuring the amount of a substance. The definition of a mole comes from how many particles (atoms, in this case) there is in exactly 12 grams of Carbon-12. Through many years of experimentation, it has been confirmed that a mole of any substance has 6 ...
... The mole (abbreviated mol) is the base unit for measuring the amount of a substance. The definition of a mole comes from how many particles (atoms, in this case) there is in exactly 12 grams of Carbon-12. Through many years of experimentation, it has been confirmed that a mole of any substance has 6 ...
E:\My Documents\snc1d\feb12notes.wpd
... However, we can make a pretty good hypothesis for now, based on the particle theory of matter. 1) It turns out that there are two types of pure substance: a) one type can be broken down by chemical reactions into other pure substances. b) the other type cannot be broken down into other pure substanc ...
... However, we can make a pretty good hypothesis for now, based on the particle theory of matter. 1) It turns out that there are two types of pure substance: a) one type can be broken down by chemical reactions into other pure substances. b) the other type cannot be broken down into other pure substanc ...
Class 1
... Multiple-charged ions are formed if there are many ionizable sites in the molecule, as in peptides and proteins, so that the formula masses of large molecules can be determined by ESI – another big advantage over EI. Most analyzers have limits on the size of m/z that can be measured with acceptable ...
... Multiple-charged ions are formed if there are many ionizable sites in the molecule, as in peptides and proteins, so that the formula masses of large molecules can be determined by ESI – another big advantage over EI. Most analyzers have limits on the size of m/z that can be measured with acceptable ...
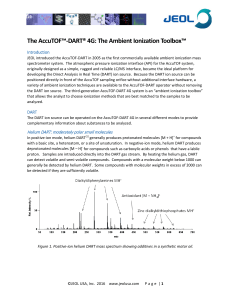
The Ambient Ionization Toolbox
... positioned directly in front of the AccuTOF sampling orifice without additional interface hardware, a variety of ambient ionization techniques are available to the AccuTOF-DART operator without removing the DART ion source. The third-generation AccuTOF-DART 4G system is an “ambient ionization toolbo ...
... positioned directly in front of the AccuTOF sampling orifice without additional interface hardware, a variety of ambient ionization techniques are available to the AccuTOF-DART operator without removing the DART ion source. The third-generation AccuTOF-DART 4G system is an “ambient ionization toolbo ...
Aerosol mass spectrometry

Aerosol mass spectrometry is the application of mass spectrometry to aerosol particles. Aerosol particles are defined as suspended solid and liquid particles with size range of 0.1 nm to 1000 μm in diameter. Aerosol particles are produce from natural and anthropogenic sources, through a variety of different process that include; wind-blown suspension, and combustion of fossil fuels and biomass. Analysis of aerosol particles is important because of their major impacts on the global climate change, visibility, regional air pollution and human health. Aerosol particles are very complex in structure and can contain thousand of different chemical compounds within a single particle. Due to this complexity the instrumentation used to analysis these particles must have the ability to separate based on size and in real-time provide information on their chemical composition. To meet these requirements for analysis, mass spectrometry instrumentation is used and they provide high sensitivity and the ability to detect a wide molecular mass range. Aerosol mass spectrometry can be divided into two categorizes; off-line and on-line. Off-line mass spectrometry is performed on collected particles. On-line mass spectrometry is performed on particles introduced in real time.