Test Review Unit 1
... 8) What is metabolism (metabolic activity)? 9) What is homeostasis? Explain how the human body maintains homeostasis (one example). 10) What is asexual reproduction? What kind of offspring does it produce (compared to the parent)? 11) What is sexual reproduction? What kind of offspring does it prod ...
... 8) What is metabolism (metabolic activity)? 9) What is homeostasis? Explain how the human body maintains homeostasis (one example). 10) What is asexual reproduction? What kind of offspring does it produce (compared to the parent)? 11) What is sexual reproduction? What kind of offspring does it prod ...
Enzymes - Solon City Schools
... Characteristics of Enzymes 1. Proteins 2. Catalysts a. Speed up chemical reactions without being used up ...
... Characteristics of Enzymes 1. Proteins 2. Catalysts a. Speed up chemical reactions without being used up ...
BL 616 Test 1 study guide. The test will probably have 20 multiple
... Ch 8 enzyme catalysis General features of enzymes: S -> P, enzyme not changed, catalysts Active (catalytic) site and what contributes Enzymes lower activation energy of reaction Six categories of enzymes; Some enzymes are RNA molecules (ribozymes) Ex. Glucokinase reaction; chymotrypsin Coenzyme exam ...
... Ch 8 enzyme catalysis General features of enzymes: S -> P, enzyme not changed, catalysts Active (catalytic) site and what contributes Enzymes lower activation energy of reaction Six categories of enzymes; Some enzymes are RNA molecules (ribozymes) Ex. Glucokinase reaction; chymotrypsin Coenzyme exam ...
Investigation of the enzymatic processes depending on the ty
... active, but there are exceptions (glycogen synthase) ...
... active, but there are exceptions (glycogen synthase) ...
practice note taking
... What type of molecule is formed when there is an uneven sharing of electrons in a covalent bond (water was the example given)? What are the building blocks of protein? An attraction between substances of the same kind ...
... What type of molecule is formed when there is an uneven sharing of electrons in a covalent bond (water was the example given)? What are the building blocks of protein? An attraction between substances of the same kind ...
CHAPTER 5 Energy and Life.
... Heat, Acidity and Enzyme concentration affect Enzyme function. If the human body temperature reaches 112 degrees F many enzymes Are destroyed. Even a temperature of 105 degrees F affects enzymes. Eating an all protein diet can affect the acidity of the blood causing Enzyme problems. Pepsin Enzyme is ...
... Heat, Acidity and Enzyme concentration affect Enzyme function. If the human body temperature reaches 112 degrees F many enzymes Are destroyed. Even a temperature of 105 degrees F affects enzymes. Eating an all protein diet can affect the acidity of the blood causing Enzyme problems. Pepsin Enzyme is ...
Energy - My CCSD
... D. Every enzyme catalyzes only one reaction or one type of reaction E. Enzymes …. 1. break down toxins (a lot in liver) 2. speed up digestion ...
... D. Every enzyme catalyzes only one reaction or one type of reaction E. Enzymes …. 1. break down toxins (a lot in liver) 2. speed up digestion ...
Organic Molecules Study Guide: Substance Basic Structure
... Which organic molecule is known for insulation? _______lipids__________ Which organic molecule is found in cellular membranes? ________lipids________ Which organic molecule helps with protein synthesis and carries genetic information? __Nucleic ...
... Which organic molecule is known for insulation? _______lipids__________ Which organic molecule is found in cellular membranes? ________lipids________ Which organic molecule helps with protein synthesis and carries genetic information? __Nucleic ...
Enzyme Notes
... Lower energy barriers for the reaction to happen at an increased rate (catalyst); not used up during the reaction ◦ activation energy (energy required to get reaction going) based on how difficult it is to break the chemical bonds ...
... Lower energy barriers for the reaction to happen at an increased rate (catalyst); not used up during the reaction ◦ activation energy (energy required to get reaction going) based on how difficult it is to break the chemical bonds ...
Document
... Digestive Enzymes: are used in the lumen of the GI tract to break down complex molecules into absorbable subunits Enzymes are biological catalysts which increase the rate of a chemical reaction without themselves becoming part of the product: ...
... Digestive Enzymes: are used in the lumen of the GI tract to break down complex molecules into absorbable subunits Enzymes are biological catalysts which increase the rate of a chemical reaction without themselves becoming part of the product: ...
12 Enzymes 9 28 05
... (a) Allosteric activators and inhibitors. In the cell, activators and inhibitors dissociate when at low concentrations. The enzyme can then oscillate again. ...
... (a) Allosteric activators and inhibitors. In the cell, activators and inhibitors dissociate when at low concentrations. The enzyme can then oscillate again. ...
Name: Date: Per: ______ EXAM STUDY GUIDE
... 13. What happens in glycolysis? Why is this thought to be an ancient form of respiration? ...
... 13. What happens in glycolysis? Why is this thought to be an ancient form of respiration? ...
Biochemistry
... a) proteins with a 3-D shape. b) shape determines the substrate for the enzyme c) Substrates- substances that enzymes act upon. Lipase acts on lipids. Maltase acts on maltose. Protease acts on proteins. Lactase works on Lactose . ...
... a) proteins with a 3-D shape. b) shape determines the substrate for the enzyme c) Substrates- substances that enzymes act upon. Lipase acts on lipids. Maltase acts on maltose. Protease acts on proteins. Lactase works on Lactose . ...
CATALYSIS OF BIOCHEMICAL REACTIONS
... 1. A and B together have some potential energy (in chemical bonds) and kinetic energy (in motion). 2. A and B collide; collision distorts or stresses bonds to the point where they can rearrange electrons; generally, this requires more potential energy (since without stress, one expects electrons to ...
... 1. A and B together have some potential energy (in chemical bonds) and kinetic energy (in motion). 2. A and B collide; collision distorts or stresses bonds to the point where they can rearrange electrons; generally, this requires more potential energy (since without stress, one expects electrons to ...
2.3: Carbon-Based Molecules
... • Enzymes are mostly proteins • Make reactions that happen in cells possible – Lower activation energy and speed up reaction rate ...
... • Enzymes are mostly proteins • Make reactions that happen in cells possible – Lower activation energy and speed up reaction rate ...
Lect2(Enzim
... ATP are hydrolyzed during the reduction By contrast, in the industrial synthesis of ammonia from nitrogen and hydrogen, the conditions used are as follows: temperature 700 - 900 K, pressure 100 - 900 atmospheres, and the presence of an iron catalyst, often promoted by traces of oxides of other met ...
... ATP are hydrolyzed during the reduction By contrast, in the industrial synthesis of ammonia from nitrogen and hydrogen, the conditions used are as follows: temperature 700 - 900 K, pressure 100 - 900 atmospheres, and the presence of an iron catalyst, often promoted by traces of oxides of other met ...
Unit_biology_2_Proteins__Enzymes
... Candidates should use their skills, knowledge and understanding of how science works: ...
... Candidates should use their skills, knowledge and understanding of how science works: ...
Name
... 3. What are the four categories of organic macromolecules? 4. Which three atoms are found in all of the organic macromolecules? 5. Explain dehydration synthesis and hydrolysis reactions. 6. Draw the following molecules: fats, nucleotide, amino acid, and monosaccharide. 7. The word saccharide means s ...
... 3. What are the four categories of organic macromolecules? 4. Which three atoms are found in all of the organic macromolecules? 5. Explain dehydration synthesis and hydrolysis reactions. 6. Draw the following molecules: fats, nucleotide, amino acid, and monosaccharide. 7. The word saccharide means s ...
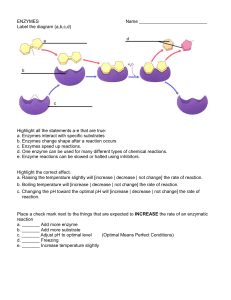
ENZYMES
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.