
guide to licensing requirements
... Dear California Building Official: In its continued efforts to promote and provide educational growth for our CALBO members and their staff, and the edification of design and construction professionals and the general public, CALBO and the CALBO State License Board Committee is pleased to present th ...
... Dear California Building Official: In its continued efforts to promote and provide educational growth for our CALBO members and their staff, and the edification of design and construction professionals and the general public, CALBO and the CALBO State License Board Committee is pleased to present th ...
Guidelines on Assessment of Applications for Prequalification
... line with the ICH guidelines (e.g., NMT 1.0% instead of NMT 0.1%). Residual solvents were included in the in-house monograph but not in the DMF. ...
... line with the ICH guidelines (e.g., NMT 1.0% instead of NMT 0.1%). Residual solvents were included in the in-house monograph but not in the DMF. ...
ASEAN Variation Guidelines For Pharmaceutical Products
... Change and/or addition of alternative manufacturer/site of drug substance [where European Pharmacopoeial Certificate of Suitability (CEP) is available] Change of batch size of drug substance [where European Pharmacopoeial Certificate of Suitability (CEP) is not available] Change of in-process contro ...
... Change and/or addition of alternative manufacturer/site of drug substance [where European Pharmacopoeial Certificate of Suitability (CEP) is available] Change of batch size of drug substance [where European Pharmacopoeial Certificate of Suitability (CEP) is not available] Change of in-process contro ...
DTP Practice Note 2 - Department of Education and Skills
... The Value per day in excess of the earliest date for substantial completion must be left blank. As all DoES projects have set project durations it is not applicable. Liquidated damages apply after the due date for substantial completion. ...
... The Value per day in excess of the earliest date for substantial completion must be left blank. As all DoES projects have set project durations it is not applicable. Liquidated damages apply after the due date for substantial completion. ...
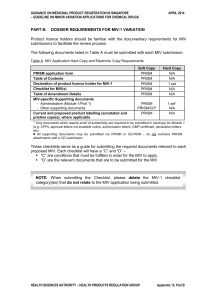
part b: dossier requirements for miv-1 variation
... 1. Synthetic route is different (for example, new intermediates are formed). 2. For a major change of the manufacturing process of a drug substance where a CEP is not available, please refer to MIV-1 B3. ...
... 1. Synthetic route is different (for example, new intermediates are formed). 2. For a major change of the manufacturing process of a drug substance where a CEP is not available, please refer to MIV-1 B3. ...
(CTD) & EUROPEAN MEDICINES
... • This document provides specification for transmitting electronic submissions using eCTD specifications. Details are included for transmitting the electronic submission on physical media or electronically. Section 23 Standards and Successful Document Creation. • PowerPoint presentation on standards ...
... • This document provides specification for transmitting electronic submissions using eCTD specifications. Details are included for transmitting the electronic submission on physical media or electronically. Section 23 Standards and Successful Document Creation. • PowerPoint presentation on standards ...
the active pharmaceutical ingredients starting material
... in the synthesis scheme) and on the manufacture of any reagents, solvents, catalysts, processing aids, and so forth, some of which are actually used in the final API manufacturing step, 5. Similar balance should exist between the API and any excipients that will become constituents of the final drug ...
... in the synthesis scheme) and on the manufacture of any reagents, solvents, catalysts, processing aids, and so forth, some of which are actually used in the final API manufacturing step, 5. Similar balance should exist between the API and any excipients that will become constituents of the final drug ...
IEEE APPROVES START OF FOUR WIRELESS PERSONAL AREA NETWORK STANDARDS PROJECTS
... IEEE P802.15.5™, “Recommended Practices for Mesh Topology Capability in Wireless Personal Area Networks (WPANs),” will provide an architectural framework for interoperable, stable and scaleable wireless mesh topologies for WPAN devices. Mesh topologies can extend network coverage without increasing ...
... IEEE P802.15.5™, “Recommended Practices for Mesh Topology Capability in Wireless Personal Area Networks (WPANs),” will provide an architectural framework for interoperable, stable and scaleable wireless mesh topologies for WPAN devices. Mesh topologies can extend network coverage without increasing ...
III. Non-clinical aspects
... male (25) and female (3 surgically sterile or post-menopausal) subjects were enrolled and the first twenty-four subjects were included into PK and statistical evaluation. The clinical part of the study was carried at the Anapharm Inc., Canada. Plasma concentrations of risperidone and 9-OH-risperidon ...
... male (25) and female (3 surgically sterile or post-menopausal) subjects were enrolled and the first twenty-four subjects were included into PK and statistical evaluation. The clinical part of the study was carried at the Anapharm Inc., Canada. Plasma concentrations of risperidone and 9-OH-risperidon ...
PDF Brochure
... • Flexible setup with USB connection — connect to an Epson projector* or PC/Mac computer with included software ...
... • Flexible setup with USB connection — connect to an Epson projector* or PC/Mac computer with included software ...
Investigator brochure or IMP dossier
... Summary of Product Characteristics (SmPC) may be provided instead of an IMPD as long as the product is being used for the same indication and in accordance with the marketing authorisation. 3. Format of information 3.1 Full IMPD. The sections of the IMPD should follow the structure described in the ...
... Summary of Product Characteristics (SmPC) may be provided instead of an IMPD as long as the product is being used for the same indication and in accordance with the marketing authorisation. 3. Format of information 3.1 Full IMPD. The sections of the IMPD should follow the structure described in the ...
III. Non-clinical aspects
... The batch formula, manufacturing process and in process controls were described in adequate details. Satisfactory process validation protocol for the drug products (for the 1, 2, 3, 4 mg strengths) has been provided. This protocol has been included and is satisfactory – includes description of manuf ...
... The batch formula, manufacturing process and in process controls were described in adequate details. Satisfactory process validation protocol for the drug products (for the 1, 2, 3, 4 mg strengths) has been provided. This protocol has been included and is satisfactory – includes description of manuf ...
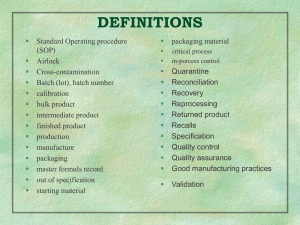
No Slide Title
... standards appropriate to their intended use and as required by the marketing authorization. QUALITY CONTROL: It is that part of GMP concerned with sampling, specifications and testing and with the organization, documentation, and release procedures which ensure that the necessary and relevant tests ...
... standards appropriate to their intended use and as required by the marketing authorization. QUALITY CONTROL: It is that part of GMP concerned with sampling, specifications and testing and with the organization, documentation, and release procedures which ensure that the necessary and relevant tests ...
SPAWAR AFCEA Cybersecurity Brf (RDML Ailes)
... together & not organized or aligned for effective interoperability ...
... together & not organized or aligned for effective interoperability ...
Quality Unit - Skills Commons
... once a product has been manufactured it must be tested to ensure it meets the specifications for safety, purity, efficacy, and quality test record review ensures that the product has met parameters that are important relative to the product quality. Any results that are outside of the specificat ...
... once a product has been manufactured it must be tested to ensure it meets the specifications for safety, purity, efficacy, and quality test record review ensures that the product has met parameters that are important relative to the product quality. Any results that are outside of the specificat ...
SOP for development of an Investigator Brochure or IMP dossier
... considerable discrepancy between different EU documents as to what is actually required. The IMPD is the most comprehensive of the two and has been accepted without an IB by the MHRA for the first use in the EU of a new vaccine in a recent Hill group trial (VAC030). An IMPD should therefore be suppl ...
... considerable discrepancy between different EU documents as to what is actually required. The IMPD is the most comprehensive of the two and has been accepted without an IB by the MHRA for the first use in the EU of a new vaccine in a recent Hill group trial (VAC030). An IMPD should therefore be suppl ...
Nikita Deshpande • 732-762
... • Review ANDAs, annual product reports, annual product reviews, and drug product labeling for FDA submissions. • Evaluate product complaints for filing Field Alert Reports (FARs) with the FDA. • Investigate product quality complaints, out of specification results (OOS), out of trend (OOT) results, a ...
... • Review ANDAs, annual product reports, annual product reviews, and drug product labeling for FDA submissions. • Evaluate product complaints for filing Field Alert Reports (FARs) with the FDA. • Investigate product quality complaints, out of specification results (OOS), out of trend (OOT) results, a ...
Progress 4GL to C#.NET
... Automated Conversion Advantages of automated solution over the manual conversion: ● Minimizes Human Errors and Reduces Testing In general, manual conversion is a routine and low productive task (UI forms, controls, business logic i.e.) that leads to high probability of human errors, may produce dif ...
... Automated Conversion Advantages of automated solution over the manual conversion: ● Minimizes Human Errors and Reduces Testing In general, manual conversion is a routine and low productive task (UI forms, controls, business logic i.e.) that leads to high probability of human errors, may produce dif ...
Tobacco Control Health Needs Assessment Norfolk 2014
... • To address the issues of disparity of terms between: o Providers: e.g. GP and Pharmacies o Geography: i.e. the former PCT areas of Norfolk and Gt Yarmouth & Waveney. ...
... • To address the issues of disparity of terms between: o Providers: e.g. GP and Pharmacies o Geography: i.e. the former PCT areas of Norfolk and Gt Yarmouth & Waveney. ...
data sheet
... requirements and hereby notifies the user that it has not tested or determined this material’s suitability or safety for use in any application. The user is responsible to adequately test and determine the safety and suitability for their application and NuSil Technology makes no warranty concerning ...
... requirements and hereby notifies the user that it has not tested or determined this material’s suitability or safety for use in any application. The user is responsible to adequately test and determine the safety and suitability for their application and NuSil Technology makes no warranty concerning ...
Document
... Although Belden makes every reasonable effort to ensure their accuracy at the time of this publication, information and specifications described herein are subject to error or omission and to change without notice, and the listing of such information and specifications does not ensure product availa ...
... Although Belden makes every reasonable effort to ensure their accuracy at the time of this publication, information and specifications described herein are subject to error or omission and to change without notice, and the listing of such information and specifications does not ensure product availa ...
6000UH Technical Data Sheet (Metric)
... Although Belden makes every reasonable effort to ensure their accuracy at the time of this publication, information and specifications described herein are subject to error or omission and to change without notice, and the listing of such information and specifications does not ensure product availa ...
... Although Belden makes every reasonable effort to ensure their accuracy at the time of this publication, information and specifications described herein are subject to error or omission and to change without notice, and the listing of such information and specifications does not ensure product availa ...
AGENCE MEDICAMENT [DRUG AGENCY] Evaluation Department
... - VEINEVA 600 mg, tablet This drug is a generic of DIOVENOR 600 mg, tablet. CLARIFICATIONS - validation of the manufacturing process must be supplied on two other pilot lots (IIB ...
... - VEINEVA 600 mg, tablet This drug is a generic of DIOVENOR 600 mg, tablet. CLARIFICATIONS - validation of the manufacturing process must be supplied on two other pilot lots (IIB ...


















![AGENCE MEDICAMENT [DRUG AGENCY] Evaluation Department](http://s1.studyres.com/store/data/008881263_1-211627a141e215023c3fd07171ec66f7-300x300.png)