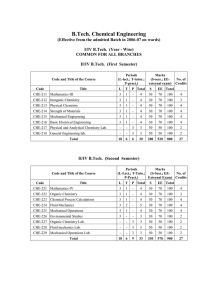
Chapter 8 Refrigeration, Heat Pump, And Power Cycles
... “high enough,” we may be able to utilize some of that energy to produce additional useful work. Note that in this example the temperature at the compressor exit/combustor inlet is 745 ºF = 396 ºC. This suggests that if we insert a heat exchanger (called a regenerator) into this cycle with the turbin ...
... “high enough,” we may be able to utilize some of that energy to produce additional useful work. Note that in this example the temperature at the compressor exit/combustor inlet is 745 ºF = 396 ºC. This suggests that if we insert a heat exchanger (called a regenerator) into this cycle with the turbin ...
Chapter 19
... A, where PA = 100 kPa, VA = 1.00 m3, and TA = 300 K. The gas is first compressed adiabatically to state B (PB = 200 kPa). The gas is then further compressed from point B to point C (VC = 0.50 m3) in an isothermal process. (a) Determine VB. (b) Calculate the work done on the gas for the whole process ...
... A, where PA = 100 kPa, VA = 1.00 m3, and TA = 300 K. The gas is first compressed adiabatically to state B (PB = 200 kPa). The gas is then further compressed from point B to point C (VC = 0.50 m3) in an isothermal process. (a) Determine VB. (b) Calculate the work done on the gas for the whole process ...
CHAPTER 3 - RIT
... 3-1C (a) If the lateral surfaces of the rod are insulated, the heat transfer surface area of the cylindrical rod is the bottom or the top surface area of the rod, As D 2 / 4 . (b) If the top and the bottom surfaces of the rod are insulated, the heat transfer area of the rod is the lateral surface ...
... 3-1C (a) If the lateral surfaces of the rod are insulated, the heat transfer surface area of the cylindrical rod is the bottom or the top surface area of the rod, As D 2 / 4 . (b) If the top and the bottom surfaces of the rod are insulated, the heat transfer area of the rod is the lateral surface ...
Heat Exchangers Lecture
... • Heat transfer in a heat exchanger usually involves convection in each fluid and conduction through the wall separating the two fluids. Thus it is convenient, in heat exchangers analysis, to work with an overall heat transfer coefficient U that accounts for the contribution of all these effects on ...
... • Heat transfer in a heat exchanger usually involves convection in each fluid and conduction through the wall separating the two fluids. Thus it is convenient, in heat exchangers analysis, to work with an overall heat transfer coefficient U that accounts for the contribution of all these effects on ...
Document
... According to the first law of thermodynamics, heat and work are related through the “internal energy” of a system and generally cannot be interconverted. However, we can ask the question: How many times does a person need to lift a 500 N barbell a height of 2 m to correspond to 2000 Calories (1 Calo ...
... According to the first law of thermodynamics, heat and work are related through the “internal energy” of a system and generally cannot be interconverted. However, we can ask the question: How many times does a person need to lift a 500 N barbell a height of 2 m to correspond to 2000 Calories (1 Calo ...
Inexistence of equilibrium states at absolute negative temperatures
... where Ω(E, N, X) is the number of “microscopic” states of a system with N atoms (or molecules) with internal energy E and a given value of the extensive parameters X. Thus, S will be monotonous in E if the energy spectrum is unbounded from above. We expect it to be bounded from below by the Ground ...
... where Ω(E, N, X) is the number of “microscopic” states of a system with N atoms (or molecules) with internal energy E and a given value of the extensive parameters X. Thus, S will be monotonous in E if the energy spectrum is unbounded from above. We expect it to be bounded from below by the Ground ...
Influence of natural convection in a porous medium when producing
... the side. In the context of geothermal energy extraction, we are interested in how the convection currents transport heat when a sealed borehole containing cold fluid extracts heat from the porous medium; also known as a borehole heat exchanger. Using pseudospectral methods together with domain decom ...
... the side. In the context of geothermal energy extraction, we are interested in how the convection currents transport heat when a sealed borehole containing cold fluid extracts heat from the porous medium; also known as a borehole heat exchanger. Using pseudospectral methods together with domain decom ...
editable version
... obtain physician order for petroleum jelly gauze or skin barrier to prevent irritation of surrounding skin. Limit the use of Dakin’s solution < 10 days.] 0.25% Acetic Acid Solution 1. Sterilize a large, clean jar that has a screw-top cap. a. Place open jar upside down and the cap in a pan of boiling ...
... obtain physician order for petroleum jelly gauze or skin barrier to prevent irritation of surrounding skin. Limit the use of Dakin’s solution < 10 days.] 0.25% Acetic Acid Solution 1. Sterilize a large, clean jar that has a screw-top cap. a. Place open jar upside down and the cap in a pan of boiling ...
CALORPLAST Heat Exchangers
... m = Quantity of fluid to be heated or cooled in lbs per hour (incorporates rate of heat-up/cool-down). Cp = Heat capacity of the fluid to be heated or cooled (consult factory or assume value of 1 BTU/lb°F). T = Difference between initial and final temperature of tank ...
... m = Quantity of fluid to be heated or cooled in lbs per hour (incorporates rate of heat-up/cool-down). Cp = Heat capacity of the fluid to be heated or cooled (consult factory or assume value of 1 BTU/lb°F). T = Difference between initial and final temperature of tank ...
Chapter 4
... All modalities lose some heat this way Evaporation Change from liquid to solid state ...
... All modalities lose some heat this way Evaporation Change from liquid to solid state ...
chapter 3 - UniMAP Portal
... Predict the pressure of nitrogen gas at T=175 K and v=0.00375 m3/kg on the basis of (a) the ideal gas equation of state and (b) the van der Waals equation of state, (c) the Beattie-Bridgeman equation of state and (d) the Benedict-Webb-Rubin equation of state. Compare the values obtained to the exper ...
... Predict the pressure of nitrogen gas at T=175 K and v=0.00375 m3/kg on the basis of (a) the ideal gas equation of state and (b) the van der Waals equation of state, (c) the Beattie-Bridgeman equation of state and (d) the Benedict-Webb-Rubin equation of state. Compare the values obtained to the exper ...
chapter-11 ncert solutions
... temperature axis at 10 atm. The fusion and boiling points are given by the intersection point where this parallel line cuts the fusion and vaporisation curves. (d) If CO2 is heated to 70°C and compressed isothermally, then it will not exhibit any transition to the liquid state. This is because 70°C ...
... temperature axis at 10 atm. The fusion and boiling points are given by the intersection point where this parallel line cuts the fusion and vaporisation curves. (d) If CO2 is heated to 70°C and compressed isothermally, then it will not exhibit any transition to the liquid state. This is because 70°C ...
Heat equation

The heat equation is a parabolic partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time.