The first and second law of Thermodynamics - Ole Witt
... This work is, however, reversible, since it is the precisely equal to the work which has to be done to bring the gas back to its initial state, releasing the heat Q to the external reservoir. It is rather easy to convince yourself that Wirr < Wrev. Thus if we let the external pressure Pext be less t ...
... This work is, however, reversible, since it is the precisely equal to the work which has to be done to bring the gas back to its initial state, releasing the heat Q to the external reservoir. It is rather easy to convince yourself that Wirr < Wrev. Thus if we let the external pressure Pext be less t ...
Development of a Prototype Thermal Management
... in each individual layer is removed through its own Model Geometry and Parameters individual perimeter area, then the stack height will not be limited thermally and the same planar IC could be used in The temperature profiles in the stacked layers of a 3D each layer. Epoxy ...
... in each individual layer is removed through its own Model Geometry and Parameters individual perimeter area, then the stack height will not be limited thermally and the same planar IC could be used in The temperature profiles in the stacked layers of a 3D each layer. Epoxy ...
Ch_15
... temperature 1 degree Celsius. – 1 gram of iron requires 1/8 as much energy for the same temperature increase. Therefore, water absorbs more heat than iron for the same change in temperature. Water has a higher specific heat. © 2010 Pearson Education, Inc. ...
... temperature 1 degree Celsius. – 1 gram of iron requires 1/8 as much energy for the same temperature increase. Therefore, water absorbs more heat than iron for the same change in temperature. Water has a higher specific heat. © 2010 Pearson Education, Inc. ...
Air and Conduction Cooling for 3U COTS Cards
... decision of which cooling method to use for 3U COTS cards in rugged applications. One of these trends is the increased use of multicore processors on 3U cards. Placing more processor cores on a die increases power dissipation. However, as more cores are placed on a die, the size of the die increases ...
... decision of which cooling method to use for 3U COTS cards in rugged applications. One of these trends is the increased use of multicore processors on 3U cards. Placing more processor cores on a die increases power dissipation. However, as more cores are placed on a die, the size of the die increases ...
Exercises - Net Start Class
... 47. Circle the letter that best describes the entropy of natural systems. a. Most natural systems will have a constant level of entropy. b. In the long run, the entropy will always increase. c. In all but a few cases, entropy in the long run will decrease. d. All natural systems have constant levels ...
... 47. Circle the letter that best describes the entropy of natural systems. a. Most natural systems will have a constant level of entropy. b. In the long run, the entropy will always increase. c. In all but a few cases, entropy in the long run will decrease. d. All natural systems have constant levels ...
1.1 Units of Measurement
... cold an object is. The SI unit for reporting temperature is Kelvin (K). See the comparison of the three scales: ...
... cold an object is. The SI unit for reporting temperature is Kelvin (K). See the comparison of the three scales: ...
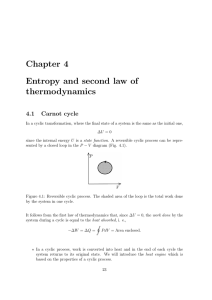
Chapter 4 Entropy and second law of thermodynamics
... As an illustration, let us consider a class in the school, where all kids sit always in the same place. This situation can be defined as ”ordered” since there is a strong correlation between the kids’ positions. For example, if Peter sits in the first row, you know straight away who is sitting behin ...
... As an illustration, let us consider a class in the school, where all kids sit always in the same place. This situation can be defined as ”ordered” since there is a strong correlation between the kids’ positions. For example, if Peter sits in the first row, you know straight away who is sitting behin ...
lab module-1
... Thermal conduction is the mode of heat transfer, which occurs in a material by virtue of a temperature gradient. A solid is chosen for the demonstration of pure conduction since both liquids and gases exhibit excessive convective heat transfer. In a practical situation, heat conduction occurs in thr ...
... Thermal conduction is the mode of heat transfer, which occurs in a material by virtue of a temperature gradient. A solid is chosen for the demonstration of pure conduction since both liquids and gases exhibit excessive convective heat transfer. In a practical situation, heat conduction occurs in thr ...
Assignment 05 A
... (One-half of the first reaction plus the other two reactions as written gives the desired overall formation reaction. Halve the heat from the first reaction and add this to the enthalpy changes for the other two reactions.) 9- Which of the following changes always results in a negative E? a) The sy ...
... (One-half of the first reaction plus the other two reactions as written gives the desired overall formation reaction. Halve the heat from the first reaction and add this to the enthalpy changes for the other two reactions.) 9- Which of the following changes always results in a negative E? a) The sy ...
Chapter 4
... All modalities lose some heat this way Evaporation Change from liquid to solid state ...
... All modalities lose some heat this way Evaporation Change from liquid to solid state ...
ABSTRACT: CFD analysis of flow and temperature
... standard of living have increased the per capita energy use and the associated pollution to an alarming level. A survey carried out at 13 most industrialized nations has shown that about 38 % of the total energy is spent for comfort applications [1]. Chen [2] strongly points out that similar aspects ...
... standard of living have increased the per capita energy use and the associated pollution to an alarming level. A survey carried out at 13 most industrialized nations has shown that about 38 % of the total energy is spent for comfort applications [1]. Chen [2] strongly points out that similar aspects ...
Chapter 1 INTRODUCTION AND BASIC CONCEPTS
... Energy Balance for Steady-Flow Systems A large number of engineering devices such as water heaters and car radiators involve mass flow in and out of a system, and are modeled as control volumes. Most control volumes are analyzed under steady operating conditions. The term steady means no change wit ...
... Energy Balance for Steady-Flow Systems A large number of engineering devices such as water heaters and car radiators involve mass flow in and out of a system, and are modeled as control volumes. Most control volumes are analyzed under steady operating conditions. The term steady means no change wit ...
Heat Transfer
... Energy Balance for Steady-Flow Systems A large number of engineering devices such as water heaters and car radiators involve mass flow in and out of a system, and are modeled as control volumes. Most control volumes are analyzed under steady operating conditions. The term steady means no change wit ...
... Energy Balance for Steady-Flow Systems A large number of engineering devices such as water heaters and car radiators involve mass flow in and out of a system, and are modeled as control volumes. Most control volumes are analyzed under steady operating conditions. The term steady means no change wit ...
Heat - FER
... Energy Balance for Steady-Flow Systems A large number of engineering devices such as water heaters and car radiators involve mass flow in and out of a system, and are modeled as control volumes. Most control volumes are analyzed under steady operating conditions. The term steady means no change wit ...
... Energy Balance for Steady-Flow Systems A large number of engineering devices such as water heaters and car radiators involve mass flow in and out of a system, and are modeled as control volumes. Most control volumes are analyzed under steady operating conditions. The term steady means no change wit ...
Heat of Sublimation - Chemwiki
... kJ/kg, then calculate the heat of sublimation for 1.00 kg of H2O(s) with the initial temperature, 273K (Hint: 273K is the solid-liquid phase change temperature and 373K is the liquid-gas phase change temperature). Using the information given in question one, calculate the heat of sublimation for 1. ...
... kJ/kg, then calculate the heat of sublimation for 1.00 kg of H2O(s) with the initial temperature, 273K (Hint: 273K is the solid-liquid phase change temperature and 373K is the liquid-gas phase change temperature). Using the information given in question one, calculate the heat of sublimation for 1. ...
Heat Transfer
... Energy Balance for Steady-Flow Systems A large number of engineering devices such as water heaters and car radiators involve mass flow in and out of a system, and are modeled as control volumes. Most control volumes are analyzed under steady operating conditions. The term steady means no change wit ...
... Energy Balance for Steady-Flow Systems A large number of engineering devices such as water heaters and car radiators involve mass flow in and out of a system, and are modeled as control volumes. Most control volumes are analyzed under steady operating conditions. The term steady means no change wit ...
1 11.8 Definition of entropy and the modern statement of the second
... equilibrium states of the systems involved in this compression, we must make sure: (i) to decrease the temperature of the gas back to its original value; (ii) to move the piston back to its original position. Suppose that we could come up with an adiabatic process to achieve these. We can then let t ...
... equilibrium states of the systems involved in this compression, we must make sure: (i) to decrease the temperature of the gas back to its original value; (ii) to move the piston back to its original position. Suppose that we could come up with an adiabatic process to achieve these. We can then let t ...
ac nanocalorimeter for measuring heat capacity of biological
... dissolved in buffered solution. This method is particularly useful for studying thermal properties of biological macromolecules in solution, since the heat capacity of macromolecules can be measured with a 10 g sample. © 2003 American Institute of Physics. 关DOI: 10.1063/1.1602958兴 ...
... dissolved in buffered solution. This method is particularly useful for studying thermal properties of biological macromolecules in solution, since the heat capacity of macromolecules can be measured with a 10 g sample. © 2003 American Institute of Physics. 关DOI: 10.1063/1.1602958兴 ...
Green building characterstics
... later and mostly cooler hours of the day. Even simple cost effective measures like a whitewash or light coloured distemper on the building exterior can reduce solar heat gain by reflecting heat. Trombe walls can be constructed to significantly enhance heat storage of walls, particularly for cold and ...
... later and mostly cooler hours of the day. Even simple cost effective measures like a whitewash or light coloured distemper on the building exterior can reduce solar heat gain by reflecting heat. Trombe walls can be constructed to significantly enhance heat storage of walls, particularly for cold and ...
Heat wave

A heat wave is a prolonged period of excessively hot weather, which may be accompanied by high humidity, especially in oceanic climate countries. While definitions vary, a heat wave is measured relative to the usual weather in the area and relative to normal temperatures for the season. Temperatures that people from a hotter climate consider normal can be termed a heat wave in a cooler area if they are outside the normal climate pattern for that area.The term is applied both to routine weather variations and to extraordinary spells of heat which may occur only once a century. Severe heat waves have caused catastrophic crop failures, thousands of deaths from hyperthermia, and widespread power outages due to increased use of air conditioning. A heat wave is considered extreme weather, and a danger because heat and sunlight may overheat the human body.