Ch100_ch4
... much hydrogen gas (in grams) is reacted in this process? a. First write out the basic chemical reaction b. Draw a table around this reaction, separating each substance ...
... much hydrogen gas (in grams) is reacted in this process? a. First write out the basic chemical reaction b. Draw a table around this reaction, separating each substance ...
The transformation of a main sequence star into a red
... Both, the core and the shell owe their stability to a property of stars which usually is referred to as negative heat capacity. A negative heat capacity of a system means that its temperature decreases when a heat flow enters it . However, the temperature decrease has to be paid for. It can be reali ...
... Both, the core and the shell owe their stability to a property of stars which usually is referred to as negative heat capacity. A negative heat capacity of a system means that its temperature decreases when a heat flow enters it . However, the temperature decrease has to be paid for. It can be reali ...
3. Basics of Heat Transfer
... Uc is the overall heat transfer coefficient based on the cold side area, W/m2.K. Ac is the total heat transfer surface area adjacent to the cold fluid side, m2. Uh is the overall heat transfer coefficient based on the hot side area, W/m2.K. Ah is the total heat transfer surface area adjacent to the ...
... Uc is the overall heat transfer coefficient based on the cold side area, W/m2.K. Ac is the total heat transfer surface area adjacent to the cold fluid side, m2. Uh is the overall heat transfer coefficient based on the hot side area, W/m2.K. Ah is the total heat transfer surface area adjacent to the ...
Humphrey, Tammy - Quantum Electronics Group
... Summary of the talk so far: • Thermoelectric energy conversion can occur reversibly if particle transport is energy selective (i.e. heat transfer can be non-isothermal but still isentropic) ...
... Summary of the talk so far: • Thermoelectric energy conversion can occur reversibly if particle transport is energy selective (i.e. heat transfer can be non-isothermal but still isentropic) ...
II-4
... • E.g. in solid conductors each atom shares some of its electrons, those least strongly bounded, with the other atoms. • In zero electric field these electrons normally move chaotically at very high speeds and undergo frequent collisions with the array of atoms of the solid. It resembles thermal mov ...
... • E.g. in solid conductors each atom shares some of its electrons, those least strongly bounded, with the other atoms. • In zero electric field these electrons normally move chaotically at very high speeds and undergo frequent collisions with the array of atoms of the solid. It resembles thermal mov ...
Procedure
... aqueous NH3 with aqueous HCl to form aqueous ammonium chloride. 2. To use calorimetry to measure the heat of solution of ammonium chloride. 3. To calculate the heat of formation of solid ammonium chloride using these data and the known heats of formation of NH3 and HCl solutions. Discussion: When a ...
... aqueous NH3 with aqueous HCl to form aqueous ammonium chloride. 2. To use calorimetry to measure the heat of solution of ammonium chloride. 3. To calculate the heat of formation of solid ammonium chloride using these data and the known heats of formation of NH3 and HCl solutions. Discussion: When a ...
Latent Heat of Vaporization and Speci c Heat - Physlab
... We know that molecules are always on the move as they have kinetic energy, but the question is, how is this energy shared? James Clerk Maxwell solved this problem for a large number of molecules. He said that energy is equally divided in all the directions a molecule is free to move. The average ene ...
... We know that molecules are always on the move as they have kinetic energy, but the question is, how is this energy shared? James Clerk Maxwell solved this problem for a large number of molecules. He said that energy is equally divided in all the directions a molecule is free to move. The average ene ...
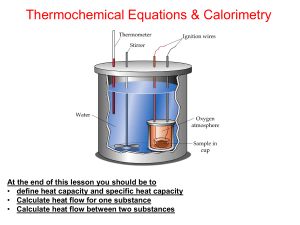
Thermochemistry Lesson 2
... At the end of this lesson you should be to • define calorimeter and calorimetry • Know five (5) ways to determine enthalpy change for a reaction • Do calculations for enthaphy of reaction using calorimetry ...
... At the end of this lesson you should be to • define calorimeter and calorimetry • Know five (5) ways to determine enthalpy change for a reaction • Do calculations for enthaphy of reaction using calorimetry ...
C060
... the same power is distributed in each case, the power per groove is more in the 3 V-groove configuration. As more power is dissipated per Vgroove here, the temperature rise in each V groove is higher. Since for this analysis, the material properties have been assumed to remain constant with temperat ...
... the same power is distributed in each case, the power per groove is more in the 3 V-groove configuration. As more power is dissipated per Vgroove here, the temperature rise in each V groove is higher. Since for this analysis, the material properties have been assumed to remain constant with temperat ...
AP Physics - Thermodynamics
... U is the change in internal energy of the system, Q is the heat added to the system, and W is the work added to the system (or done on the system). Q is positive when it is added to the system and negative if it is taken out of the system. W is positive if it is added to the system and negative if ...
... U is the change in internal energy of the system, Q is the heat added to the system, and W is the work added to the system (or done on the system). Q is positive when it is added to the system and negative if it is taken out of the system. W is positive if it is added to the system and negative if ...
Epoxies and Glass Transition Temperature
... have high Tg values. An exception is the Master Bond family of EP36 systems, which are B-staged epoxies. In B-staging, the resin and hardener are mixed, and a heat cure is initiated, but the reaction is arrested by quenching or cooling while the adhesive is still fusible and soluble. The system is p ...
... have high Tg values. An exception is the Master Bond family of EP36 systems, which are B-staged epoxies. In B-staging, the resin and hardener are mixed, and a heat cure is initiated, but the reaction is arrested by quenching or cooling while the adhesive is still fusible and soluble. The system is p ...
Document
... • Temperature tells us whether or not two systems will change when brought into thermal contact with one another. • A thermometer is just a very small system with only one state variable. ...
... • Temperature tells us whether or not two systems will change when brought into thermal contact with one another. • A thermometer is just a very small system with only one state variable. ...
heat vs temp student sheet
... Temperature is a property directly proportional to the kinetic energy of a substance. Temperature is measured using a thermometer and has units of K, oC or oF. Temperature is represented by the symbol ΔT or T. ΔT is calculated as Tfinal – Tinitial. ...
... Temperature is a property directly proportional to the kinetic energy of a substance. Temperature is measured using a thermometer and has units of K, oC or oF. Temperature is represented by the symbol ΔT or T. ΔT is calculated as Tfinal – Tinitial. ...
ENGR 7901 - Heat Transfer II 1 Introduction 2 The Flat Plate
... and transversly are inline. Staggered tube arrays have every other row offset usually by one half of the tube to tube spacing. In such applications, the heat transfer coefficient and hence the Nusselt number degrades as a result of the complex flow arrangements and warming of the fluid. Since the cy ...
... and transversly are inline. Staggered tube arrays have every other row offset usually by one half of the tube to tube spacing. In such applications, the heat transfer coefficient and hence the Nusselt number degrades as a result of the complex flow arrangements and warming of the fluid. Since the cy ...
JIF 314 Thermodynamics
... A generic natural process usually involve the conversion of forms of energy. In particular, the conversion of energy form from work into internal energy, Wf = DU can only happen in the presence of an agent of dissipation, e.g. friction or viscosity. Say, consider a moving object with kinetic energy ...
... A generic natural process usually involve the conversion of forms of energy. In particular, the conversion of energy form from work into internal energy, Wf = DU can only happen in the presence of an agent of dissipation, e.g. friction or viscosity. Say, consider a moving object with kinetic energy ...
LAW: The first law of thermodynamics states that the total energy in
... effective diet method. Determine the internal energy change this would cause. What if you used ice cubes instead of water? DEFINITION: The latent heat is the specific enthalpy change associated with a change in state of aggregation (phase). NOTE: The latent heat is often known only at a single tempe ...
... effective diet method. Determine the internal energy change this would cause. What if you used ice cubes instead of water? DEFINITION: The latent heat is the specific enthalpy change associated with a change in state of aggregation (phase). NOTE: The latent heat is often known only at a single tempe ...
LAW: The first law of thermodynamics states that the total energy in
... • Identify, formulate, and solve simple engineering problems (such as expansion/ compression and power cycles) • Describe the molecular basis for internal energy, heat transfer, work, and heat capacity • Explain the difference between a reversible and irreversible process • Distinguish between rever ...
... • Identify, formulate, and solve simple engineering problems (such as expansion/ compression and power cycles) • Describe the molecular basis for internal energy, heat transfer, work, and heat capacity • Explain the difference between a reversible and irreversible process • Distinguish between rever ...
GeoT*SOL® Exploiting the Earth`s Sustainable Energy Supply
... the entire heat pump system over one year, the program then determines the r espective SPF. With this parameter and additional results from the minute-step simulation, GeoT*SOL® basic evaluates the economic efficiency of a system by establishing a ratio of heat price to anticipated service life. Th ...
... the entire heat pump system over one year, the program then determines the r espective SPF. With this parameter and additional results from the minute-step simulation, GeoT*SOL® basic evaluates the economic efficiency of a system by establishing a ratio of heat price to anticipated service life. Th ...
calorimetry
... heating the metal to a known temperature, placing it into a calorimeter containing a measured amount of water at a known temperature, and measuring the final equilibrium temperature. This process assumes no heat is lost to the calorimeter. By assuming that the heat lost by the metal is equal the hea ...
... heating the metal to a known temperature, placing it into a calorimeter containing a measured amount of water at a known temperature, and measuring the final equilibrium temperature. This process assumes no heat is lost to the calorimeter. By assuming that the heat lost by the metal is equal the hea ...
HNRS 227 Lecture #2 Chapters 2 and 3
... weighs more per volume than the warmer air and pushes the warmer air out of the way as it sinks down to its lowest level. The warmer, less dense air sits on top of the cooler air because it weighs less per volume. ...
... weighs more per volume than the warmer air and pushes the warmer air out of the way as it sinks down to its lowest level. The warmer, less dense air sits on top of the cooler air because it weighs less per volume. ...