DiagnosticTest
... What is the percentage of oxygen in expired air when a person is resting? a. 8% b. 12% c. 16% d. 20% ...
... What is the percentage of oxygen in expired air when a person is resting? a. 8% b. 12% c. 16% d. 20% ...
Done by: Terence Lee (27) - ScienceIMPORTANTRCYJTLCEC
... purchase from an art store and we constructed the box. We split the components of the report into equal portions to do and then we combined them. The report was finalized after compiling our various works. ...
... purchase from an art store and we constructed the box. We split the components of the report into equal portions to do and then we combined them. The report was finalized after compiling our various works. ...
Unit Test: Atmospheric Forces
... reaching Earth. 6. What portion of the electromagnetic spectrum is LEAST absorbed by the layers of the atmosphere before reaching Earth. 7. What happens to solar radiation that is not reflected back into the atmosphere or absorbed by the Earth’s surface. 8. List the parts of the global wind belt 9. ...
... reaching Earth. 6. What portion of the electromagnetic spectrum is LEAST absorbed by the layers of the atmosphere before reaching Earth. 7. What happens to solar radiation that is not reflected back into the atmosphere or absorbed by the Earth’s surface. 8. List the parts of the global wind belt 9. ...
Heat Recovery for Commercial Buildings
... Loads are shown for an open plan office. Mass flow rate of air m = 7.41kg/s. The OAC, RAC, SAT are plotted on the psychometric chart. The recuperator has an efficiency of 70%. The cooling coil load is calculated using the formula Qcc = m(Δh)..kW. Cooling Coil load without HR = 7.41x (68. ...
... Loads are shown for an open plan office. Mass flow rate of air m = 7.41kg/s. The OAC, RAC, SAT are plotted on the psychometric chart. The recuperator has an efficiency of 70%. The cooling coil load is calculated using the formula Qcc = m(Δh)..kW. Cooling Coil load without HR = 7.41x (68. ...
Thermochemistry: Energy Flow and Chemical
... Thermochemistry: Energy Flow and Chemical Change 6.1 Forms of Energy and Their Interconversion When energy is transferred from one object to another, it appears as work and/or heat ∆E = Efinal – Einitial = Eproducts – Ereactants ∆ – refers to the final state of the system minus the initial state Bec ...
... Thermochemistry: Energy Flow and Chemical Change 6.1 Forms of Energy and Their Interconversion When energy is transferred from one object to another, it appears as work and/or heat ∆E = Efinal – Einitial = Eproducts – Ereactants ∆ – refers to the final state of the system minus the initial state Bec ...
Optimal heating and cooling strategies for heat exchanger design
... heat exchanger design which reduces the entropy production can in principle provide additional useful work somewhere else in the plant. The following extensive energy consumers are often equipped to exploit this available work: factories with cogeneration capabilities, distillation plants driven by ...
... heat exchanger design which reduces the entropy production can in principle provide additional useful work somewhere else in the plant. The following extensive energy consumers are often equipped to exploit this available work: factories with cogeneration capabilities, distillation plants driven by ...
ME6301- ENGINEERING THERMODYNAMICS UNIT – I BASIC
... phases coexist in equilibrium. At the critical point the liquid and vapour phases are indistinguishable i.e. Liquid is directly converted into vapour. 7. One kg of steam at 10 bar has an enthalpy of 2500 kJ/kg. Find its quality.(A/M 2008) ...
... phases coexist in equilibrium. At the critical point the liquid and vapour phases are indistinguishable i.e. Liquid is directly converted into vapour. 7. One kg of steam at 10 bar has an enthalpy of 2500 kJ/kg. Find its quality.(A/M 2008) ...
Chapter 5.doc
... In general, to determine the Nusselt number it is necessary to determine the velocity and temperature distribution. ...
... In general, to determine the Nusselt number it is necessary to determine the velocity and temperature distribution. ...
PHYSICS 1030L LAB: Heat of Fusion
... In this equation, ΔQ is the amount of heat flow, as before, m is the mass of the object, and L is an intrinsic constant of the material. If the material is melting (i.e. changing from a solid to a liquid), then L is known as the Latent Heat of Fusion and is written as Lf . If the object is being vapo ...
... In this equation, ΔQ is the amount of heat flow, as before, m is the mass of the object, and L is an intrinsic constant of the material. If the material is melting (i.e. changing from a solid to a liquid), then L is known as the Latent Heat of Fusion and is written as Lf . If the object is being vapo ...
24. Conduction Cooling for Chassis and Circuit Boards
... electronic system is operating, the electronic components are its hottest parts. To control the hot spot component temperatures, the heat flow path must be controlled. If this is not done properly, the component temperatures are forced to rise in an attempt to balance the heat flow. Eventually, the ...
... electronic system is operating, the electronic components are its hottest parts. To control the hot spot component temperatures, the heat flow path must be controlled. If this is not done properly, the component temperatures are forced to rise in an attempt to balance the heat flow. Eventually, the ...
Summary of Heat Transfer
... Equivalent heat conduction coefficient: ke Equivalent conduction resistance of air layer: ...
... Equivalent heat conduction coefficient: ke Equivalent conduction resistance of air layer: ...
HNRS 227 Lecture #2 Chapters 2 and 3
... The 10 pounds of ice provide more cooling because as the ice undergoes the phase change into water, it absorbs heat. Ten pounds of ice water simply absorbs heat according to the value of its specific heat until it reaches room temperature and therefore absorbs less heat. ...
... The 10 pounds of ice provide more cooling because as the ice undergoes the phase change into water, it absorbs heat. Ten pounds of ice water simply absorbs heat according to the value of its specific heat until it reaches room temperature and therefore absorbs less heat. ...
HNRS 227 Lecture #2 Chapters 2 and 3
... The 10 pounds of ice provide more cooling because as the ice undergoes the phase change into water, it absorbs heat. Ten pounds of ice water simply absorbs heat according to the value of its specific heat until it reaches room temperature and therefore absorbs less heat. ...
... The 10 pounds of ice provide more cooling because as the ice undergoes the phase change into water, it absorbs heat. Ten pounds of ice water simply absorbs heat according to the value of its specific heat until it reaches room temperature and therefore absorbs less heat. ...
strategies in thermal regulation - Evans Laboratory: Environmental
... 2.) at higher temperatures the destructive effects of temperature take over and rates of activity decline ...
... 2.) at higher temperatures the destructive effects of temperature take over and rates of activity decline ...
Boddeker`s Ch 16 Temperature and Heat (PHY122)
... So we say the “zero” point for the Fahrenheit scale is 32 °F… 37 °C uses the “small” scale I think most people will agree this is a weird let’s go to the “BIG” scale “zero” point 37 °C * 9/5 = 66.6 F° How can we bring this “zero” point to zero? o Ans: Subtract 32 °F But the “zero” point of t ...
... So we say the “zero” point for the Fahrenheit scale is 32 °F… 37 °C uses the “small” scale I think most people will agree this is a weird let’s go to the “BIG” scale “zero” point 37 °C * 9/5 = 66.6 F° How can we bring this “zero” point to zero? o Ans: Subtract 32 °F But the “zero” point of t ...
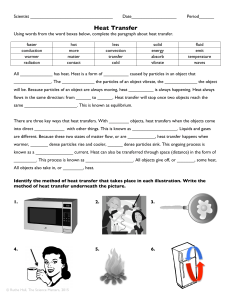
Heat Transfer - Granville County Public Schools
... All _____________ has heat. Heat is a form of __________ caused by particles in an object that _______________. The _____________ the particles of an object vibrate, the _____________ the object will be. Because particles of an object are always moving, heat __________ is always happening. Heat alwa ...
... All _____________ has heat. Heat is a form of __________ caused by particles in an object that _______________. The _____________ the particles of an object vibrate, the _____________ the object will be. Because particles of an object are always moving, heat __________ is always happening. Heat alwa ...
Chapter 12 Study Guide - School District of La Crosse
... 12.2 CHANGE OF STATE AND LAWS OF THERMODYNAMICS Change of State If the temperature of a solid is raised, it changes to a(n)______ and then to a(n)_______. This occurs Because a(n)_________ in thermal energy of a solid increases the________and__________ energies of the Particles. As the solid is heat ...
... 12.2 CHANGE OF STATE AND LAWS OF THERMODYNAMICS Change of State If the temperature of a solid is raised, it changes to a(n)______ and then to a(n)_______. This occurs Because a(n)_________ in thermal energy of a solid increases the________and__________ energies of the Particles. As the solid is heat ...
sensory neurons
... generate much heat Their muscles generate heat when active, but since most ectotherms lack effective body insulation, their body heat is easily lost to the environment ...
... generate much heat Their muscles generate heat when active, but since most ectotherms lack effective body insulation, their body heat is easily lost to the environment ...
Chapter 20: Vertebrates
... All have an endoskeleton surrounded by muscles and skin Vertebral column (backbone) is the main part of the skeleton Adapting to life on land led to changes in ...
... All have an endoskeleton surrounded by muscles and skin Vertebral column (backbone) is the main part of the skeleton Adapting to life on land led to changes in ...
• Heating foods • Moist-heat method • Dry
... Example – tough cut of meat is usually cooked by moist-heat method • The muscle portion of most meat, poultry, and fish is composed of 75% water and 20% protein. The ability of these items to hold water and contain fat affects their juiciness. • Collagen, an important protein found in meat and poult ...
... Example – tough cut of meat is usually cooked by moist-heat method • The muscle portion of most meat, poultry, and fish is composed of 75% water and 20% protein. The ability of these items to hold water and contain fat affects their juiciness. • Collagen, an important protein found in meat and poult ...
Entropy - BYU Physics and Astronomy
... Entropy is also a measure of disorder, because disordered states can be formed in many ways. ...
... Entropy is also a measure of disorder, because disordered states can be formed in many ways. ...
Heat Chap01-001 - heat transfer 2e solutions - sztook23
... 1-3C The caloric theory is based on the assumption that heat is a fluid-like substance called the "caloric" which is a massless, colorless, odorless substance. It was abandoned in the middle of the nineteenth century after it was shown that there is no such thing as the caloric. ...
... 1-3C The caloric theory is based on the assumption that heat is a fluid-like substance called the "caloric" which is a massless, colorless, odorless substance. It was abandoned in the middle of the nineteenth century after it was shown that there is no such thing as the caloric. ...
Refrigerators and Entropy
... Configurations with large W are more probable For large N (N>100) the probability of the equal distribution configurations is enormous ...
... Configurations with large W are more probable For large N (N>100) the probability of the equal distribution configurations is enormous ...
Hyperthermia
Hyperthermia is elevated body temperature due to failed thermoregulation that occurs when a body produces or absorbs more heat than it dissipates. Extreme temperature elevation then becomes a medical emergency requiring immediate treatment to prevent disability or death.The most common causes include heat stroke and adverse reactions to drugs. The former is an acute temperature elevation caused by exposure to excessive heat, or combination of heat and humidity, that overwhelms the heat-regulating mechanisms. The latter is a relatively rare side effect of many drugs, particularly those that affect the central nervous system. Malignant hyperthermia is a rare complication of some types of general anesthesia.Hyperthermia differs from fever in that the body's temperature set point remains unchanged. The opposite is hypothermia, which occurs when the temperature drops below that required to maintain normal metabolism.