Definitions of Common Heat Transfer and Insulation Terms
... Textbook definition of K-Factor: The time rate of steady heat flow through a unit area of a homogeneous material induced by a unit temperature gradient in a direction perpendicular to that unit area. Insulation materials usually have K-Factors less than one and are reported at what is called Mean Te ...
... Textbook definition of K-Factor: The time rate of steady heat flow through a unit area of a homogeneous material induced by a unit temperature gradient in a direction perpendicular to that unit area. Insulation materials usually have K-Factors less than one and are reported at what is called Mean Te ...
View PDF
... Designers continued to wrestle with how to most efficiently provide low temperature heating water for the radiant floor heating system. Cooling overheated water wastes energy and running boilers at lower temperatures would damage the units. A concept that appeared viable was to use a heating cascade ...
... Designers continued to wrestle with how to most efficiently provide low temperature heating water for the radiant floor heating system. Cooling overheated water wastes energy and running boilers at lower temperatures would damage the units. A concept that appeared viable was to use a heating cascade ...
Thermodynamics for Materials and Metallurgical Engineers
... A variable describing a particular piece of matter is said to be extensive if its value depends on the quantity of the matter being described. For example, total heat capacity and mass are both extensive variables as opposed to intensive variables such as density, specific heat capacity, and tempera ...
... A variable describing a particular piece of matter is said to be extensive if its value depends on the quantity of the matter being described. For example, total heat capacity and mass are both extensive variables as opposed to intensive variables such as density, specific heat capacity, and tempera ...
TAREA 1. Resuelva las siguientes preguntas y problemas. Además
... The review presented so far emphasized the main concepts associated with the first law and those items that are most likely to lead to confusion in the process of analyzing an engineering problem. I was unable to discuss these points without drawing attention to their historical background: I believe ...
... The review presented so far emphasized the main concepts associated with the first law and those items that are most likely to lead to confusion in the process of analyzing an engineering problem. I was unable to discuss these points without drawing attention to their historical background: I believe ...
Heat Flow Basics, Arch264
... The easiest means of estimating heat flow through an entire building is to 1. Calculate and then list the U-value for each element (wall, roof, window, door) along with the area of that element 2. The product of each elements' area and its U-value is the heat loss coefficient for that enclosure elem ...
... The easiest means of estimating heat flow through an entire building is to 1. Calculate and then list the U-value for each element (wall, roof, window, door) along with the area of that element 2. The product of each elements' area and its U-value is the heat loss coefficient for that enclosure elem ...
Energy - Mr. Rowley - Physical Science 20
... The energy a particle has because of its motion is called kinetic energy. The energy associated with the movement of particles is called heat and thermal energy. What happens to the motion of particles as they are the temperature of a substance is increased? ...
... The energy a particle has because of its motion is called kinetic energy. The energy associated with the movement of particles is called heat and thermal energy. What happens to the motion of particles as they are the temperature of a substance is increased? ...
HEAT OF VAPORIZATION (H v )
... Solids exist in a rigid, closely packed, highly structured pattern Liquids however have no such rigid structure. As we reach the solids m.p. there is just enough energy to begin overcoming the intermolecular forces between molecules holding them together in the solid state... Molecules begin to sepa ...
... Solids exist in a rigid, closely packed, highly structured pattern Liquids however have no such rigid structure. As we reach the solids m.p. there is just enough energy to begin overcoming the intermolecular forces between molecules holding them together in the solid state... Molecules begin to sepa ...
SOLUTIONS: HOMEWORK #6
... 7-51 An aluminum block is brought into contact with an iron block in an insulated enclosure. The final equilibrium temperature and the total entropy change for this process are to be determined. Assumptions 1 Both the aluminum and the iron block are incompressible substances with constant specific h ...
... 7-51 An aluminum block is brought into contact with an iron block in an insulated enclosure. The final equilibrium temperature and the total entropy change for this process are to be determined. Assumptions 1 Both the aluminum and the iron block are incompressible substances with constant specific h ...
Thermochemistry - Piedra Vista High School
... Enthalpy (H) is used to quantify the heat flow into or out of a system in a process that occurs at constant pressure. ...
... Enthalpy (H) is used to quantify the heat flow into or out of a system in a process that occurs at constant pressure. ...
Thermodynamics
... particles in any sample above 0 K – Heat -- causes a change in the thermal energy of a sample. Flows from hot to cold ...
... particles in any sample above 0 K – Heat -- causes a change in the thermal energy of a sample. Flows from hot to cold ...
Conductive heat flow at the surface is described by Fourier`s law of
... • Our results show that the GDH1, HSC, and PSM models overestimate heat flow close to the ridge, but the differences are small. • Our model does not provide evidence that heat flow is less than 44 TW. ...
... • Our results show that the GDH1, HSC, and PSM models overestimate heat flow close to the ridge, but the differences are small. • Our model does not provide evidence that heat flow is less than 44 TW. ...
Chem Unit 3 Vocabulary
... Chapter 10 – States of Matter, pp 329-351 1 amorphous solid; 2 boiling; 3 boiling point; 4 capillary action; 5 condensation; 6 critical point; 7 critical pressure; 8 critical temperature; 9 crystal; 10 crystalline solids; 11 crystal structure; 12 deposition; 13 diffusion; 14 effusion; 15 elastic col ...
... Chapter 10 – States of Matter, pp 329-351 1 amorphous solid; 2 boiling; 3 boiling point; 4 capillary action; 5 condensation; 6 critical point; 7 critical pressure; 8 critical temperature; 9 crystal; 10 crystalline solids; 11 crystal structure; 12 deposition; 13 diffusion; 14 effusion; 15 elastic col ...
Air thermal bridges
... 3. Thermal bridge calculation in the heat flux through the building envelope Existing procedures address structural thermal bridges: linear and point in the calculation of the heat flux through the building envelope. Thermal transmittance of linear thermal bridges can be approximately substituted f ...
... 3. Thermal bridge calculation in the heat flux through the building envelope Existing procedures address structural thermal bridges: linear and point in the calculation of the heat flux through the building envelope. Thermal transmittance of linear thermal bridges can be approximately substituted f ...
Chapter 5: Thermochemistry
... temperature). Can one lose weight by drinking ice-cold beverages if the body uses up about 1 calorie per gram of water per degree Celsius (i.e. the specific heat of water = 1.00 cal/g·°C) to consume the drink? a. Calculate the energy expended (in Cal) to consume a 12-oz beer (about 355 mL) if the be ...
... temperature). Can one lose weight by drinking ice-cold beverages if the body uses up about 1 calorie per gram of water per degree Celsius (i.e. the specific heat of water = 1.00 cal/g·°C) to consume the drink? a. Calculate the energy expended (in Cal) to consume a 12-oz beer (about 355 mL) if the be ...
PS Chapter 16 - NPHSPhysicalScience
... particles in a fluid b. Hot air circulates in an oven. c. The transfer of energy by waves moving through space d. Heating coil of an electric stove glows. Go to section ...
... particles in a fluid b. Hot air circulates in an oven. c. The transfer of energy by waves moving through space d. Heating coil of an electric stove glows. Go to section ...
PS225 – Heat and thermodynamics
... b is about 4 times the spatial volume occupied by a molecule (b depends on the distance at which they “feel” each other) ...
... b is about 4 times the spatial volume occupied by a molecule (b depends on the distance at which they “feel” each other) ...
HEAT LOAD
... exhaust temperature of w / (ρ c ∆t)= qv,Φw =Φ the recirculated air used qv,Φw =Φwthe/ room (ρ c ∆t)= for heating ...
... exhaust temperature of w / (ρ c ∆t)= qv,Φw =Φ the recirculated air used qv,Φw =Φwthe/ room (ρ c ∆t)= for heating ...
Low-Temperature Heat Transfer Fluids Newer Options
... Heat transfer fluids are widely used in food processing, commercial refrigeration, geothermal, and other lowtemperature heat-transfer applications that typically operate in a temperature range from 0°F to 42°F (-18°C to 6°C). Most heat transfer fluids have lower heat-transfer efficiencies than wate ...
... Heat transfer fluids are widely used in food processing, commercial refrigeration, geothermal, and other lowtemperature heat-transfer applications that typically operate in a temperature range from 0°F to 42°F (-18°C to 6°C). Most heat transfer fluids have lower heat-transfer efficiencies than wate ...
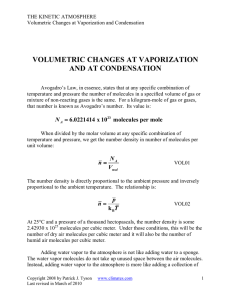
volumetric changes
... more room than before. Each water molecule takes up just as much “room” as each air molecule. Part of the problem lies in the use of the term “saturation” in discussions of humidity. It implies that there is no more space available. Actually, of course, the air and vapor molecules take up only a sma ...
... more room than before. Each water molecule takes up just as much “room” as each air molecule. Part of the problem lies in the use of the term “saturation” in discussions of humidity. It implies that there is no more space available. Actually, of course, the air and vapor molecules take up only a sma ...
energy
... law of conservation of energy (10.1) work (10.1) state function (10.1) temperature (10.2) heat (10.2) system (10.3) surroundings (10.3) exothermic (10.3) endothermic (10.3) thermodynamics (10.4) first law of thermodynamics (10.4) internal energy (10.4) calorie (10.5) joule (10.5) specific heat capac ...
... law of conservation of energy (10.1) work (10.1) state function (10.1) temperature (10.2) heat (10.2) system (10.3) surroundings (10.3) exothermic (10.3) endothermic (10.3) thermodynamics (10.4) first law of thermodynamics (10.4) internal energy (10.4) calorie (10.5) joule (10.5) specific heat capac ...
File
... Some flows are smooth and orderly while others are rather chaotic. The highly ordered fluid motion characterized by smooth streamlines is called laminar. The flow of high-viscosity fluids such as oils at low velocities is typically laminar. The highly disordered fluid motion that typically occurs at ...
... Some flows are smooth and orderly while others are rather chaotic. The highly ordered fluid motion characterized by smooth streamlines is called laminar. The flow of high-viscosity fluids such as oils at low velocities is typically laminar. The highly disordered fluid motion that typically occurs at ...
PHYSICS 1030L LAB: Heat of Fusion
... In this equation, ΔQ is the amount of heat flow, as before, m is the mass of the object, and L is an intrinsic constant of the material. If the material is melting (i.e. changing from a solid to a liquid), then L is known as the Latent Heat of Fusion and is written as Lf . If the object is being vapo ...
... In this equation, ΔQ is the amount of heat flow, as before, m is the mass of the object, and L is an intrinsic constant of the material. If the material is melting (i.e. changing from a solid to a liquid), then L is known as the Latent Heat of Fusion and is written as Lf . If the object is being vapo ...
Introduction to Thermochemistry and Specific Heat
... EX 3: What is the molar heat of combustion of propene (C3H6) if burning 3.2 g releases 156 kJ of heat? Step 1: Write the reaction equation. 2 C3H6 (g) + 9 O2 (g) 6 CO2 (g) + 6 H2O (g) C3H6 (g) + 4.5 O2 (g) 3 CO2 (g) + 3 H2O (g) • This reaction equation involves the combustion of 2 moles of C3H6 ...
... EX 3: What is the molar heat of combustion of propene (C3H6) if burning 3.2 g releases 156 kJ of heat? Step 1: Write the reaction equation. 2 C3H6 (g) + 9 O2 (g) 6 CO2 (g) + 6 H2O (g) C3H6 (g) + 4.5 O2 (g) 3 CO2 (g) + 3 H2O (g) • This reaction equation involves the combustion of 2 moles of C3H6 ...
Unit 9 Review
... Mathematically, this means that their _______________ is a constant. 12. The _______________ Gas Law permits calculation of any one term when temperature, pressure, and volume change for a gas. 13. If A and B are directly proportional and the value of A becomes 1/3 as much, what happens to the value ...
... Mathematically, this means that their _______________ is a constant. 12. The _______________ Gas Law permits calculation of any one term when temperature, pressure, and volume change for a gas. 13. If A and B are directly proportional and the value of A becomes 1/3 as much, what happens to the value ...