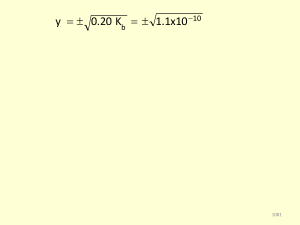
Acid‒base reaction
... reactions, devised by Gilbert N. Lewis in 1923,[12] in the same year as Brønsted–Lowry, but it was not elaborated by him until 1938.[2] Instead of defining acid–base reactions in terms of protons or other bonded substances, the Lewis definition defines a base (referred to as a Lewis base) to be a co ...
... reactions, devised by Gilbert N. Lewis in 1923,[12] in the same year as Brønsted–Lowry, but it was not elaborated by him until 1938.[2] Instead of defining acid–base reactions in terms of protons or other bonded substances, the Lewis definition defines a base (referred to as a Lewis base) to be a co ...
Acid Base Equilibria
... Weak acid: one that only partially dissociates in aqueous solution and therefore exists in the solution as a mixture of acid molecules component ions: For example, HF dissociates in water to give H+ and F-. It is a weak acid with a dissociation equation that is HF (aq) ↔ H+ (aq) + F-(aq) Note the us ...
... Weak acid: one that only partially dissociates in aqueous solution and therefore exists in the solution as a mixture of acid molecules component ions: For example, HF dissociates in water to give H+ and F-. It is a weak acid with a dissociation equation that is HF (aq) ↔ H+ (aq) + F-(aq) Note the us ...
03 Inorg. drugs with acid-base prop. IOC of С,Al, Ba,Ag
... carbonization white precipitate on the filter Sulphates A. (BrPh, SPU). Reaction with barium chloride solution in the hydrochloricacid medium. Dissolve about 45 mg of the substance to be examined in 5 ml of water R or use 5 ml of the prescribed solution. Add 1 ml of dilute hydrochloric acid R and 1 ...
... carbonization white precipitate on the filter Sulphates A. (BrPh, SPU). Reaction with barium chloride solution in the hydrochloricacid medium. Dissolve about 45 mg of the substance to be examined in 5 ml of water R or use 5 ml of the prescribed solution. Add 1 ml of dilute hydrochloric acid R and 1 ...
2013-2014
... Answers to Section A should be marked on the Multiple-choice Answer Sheet while answers to Section B should be written in the spaces provided in Question-Answer Book B. The Answer Sheet for Section A and the Question-Answer Book for Section B will be collected separately at the end of the examinatio ...
... Answers to Section A should be marked on the Multiple-choice Answer Sheet while answers to Section B should be written in the spaces provided in Question-Answer Book B. The Answer Sheet for Section A and the Question-Answer Book for Section B will be collected separately at the end of the examinatio ...
Nitrocellulose

Nitrocellulose (also: cellulose nitrate, flash paper, flash cotton, guncotton, flash string) is a highly flammable compound formed by nitrating cellulose through exposure to nitric acid or another powerful nitrating agent. When used as a propellant or low-order explosive, it was originally known as guncotton.Partially nitrated cellulose has found uses as a plastic film and in inks and wood coatings. In 1862 the first man-made plastic, nitrocellulose, (branded Parkesine) was created by Alexander Parkes from cellulose treated with nitric acid and a solvent. In 1868, American inventor John Wesley Hyatt developed a plastic material he named Celluloid, improving on Parkes' invention by plasticizing the nitrocellulose with camphor so that it could be processed into finished form and used as a photographic film. Celluloid was used by Kodak, and other suppliers, from the late 1880s as a film base in photography, X-ray films, and motion picture films, and was known as 'nitrate film'. After numerous fires caused by unstable nitrate films, safety film (cellulose acetate film) started to be used from the 1930s in the case of X-ray stock and from 1948 for motion picture film.