presentation
... • Each person will move through these stages at different rates based on their individual goals, attitude, and passion for self-development. ...
... • Each person will move through these stages at different rates based on their individual goals, attitude, and passion for self-development. ...
Introduction
... Force: Vector quantity that describes an action of one body on another [Statics] • In dynamics, force is an action that tends to cause acceleration of an object. • The SI unit of force magnitude is the newton (N). One newton is equivalent to one kilogram-meter ...
... Force: Vector quantity that describes an action of one body on another [Statics] • In dynamics, force is an action that tends to cause acceleration of an object. • The SI unit of force magnitude is the newton (N). One newton is equivalent to one kilogram-meter ...
Qatar University College of Arts and Science Department of
... Course Objectives. The aim of this course is to 1. Train the students on practicing 'statistical thinking' throughout their daily life. 2. Acquaint the students with the basic knowledge of statistics such as: Methods of descriptive statistics, Sampling techniques, the basic concepts of probability, ...
... Course Objectives. The aim of this course is to 1. Train the students on practicing 'statistical thinking' throughout their daily life. 2. Acquaint the students with the basic knowledge of statistics such as: Methods of descriptive statistics, Sampling techniques, the basic concepts of probability, ...
Introduction to Biophysical Chemistry
... Chemistry The central focus of Ch 24a emphasizes solution thermodynamics, including the concepts of energy, enthalpy, entropy, free energy, with connections to statistical thermodynamics; physical and chemical equilibrium of both ideal and real systems, including gases, solutions, and electrolytes; ...
... Chemistry The central focus of Ch 24a emphasizes solution thermodynamics, including the concepts of energy, enthalpy, entropy, free energy, with connections to statistical thermodynamics; physical and chemical equilibrium of both ideal and real systems, including gases, solutions, and electrolytes; ...
Course Home - Haldia Institute of Technology
... implications, and become familiar with their use and applications. FT301.3 Ability to predict intermolecular potential and excess property behavior of multi component systems and ability to determine rate constant of different reactions. FT301.4 Ability to understand physical transformations in pure ...
... implications, and become familiar with their use and applications. FT301.3 Ability to predict intermolecular potential and excess property behavior of multi component systems and ability to determine rate constant of different reactions. FT301.4 Ability to understand physical transformations in pure ...
Mechanics 105 chapter 7
... gravitational field, falling from yb to ya. In free fall, the work done by gravity is mg(yb-ya), which results in a change in the kinetic energy (work-kinetic energy theorem) K. This work equals -Ug, the change in the gravitational potential energy of the (earth + object) system. The earth’s kinet ...
... gravitational field, falling from yb to ya. In free fall, the work done by gravity is mg(yb-ya), which results in a change in the kinetic energy (work-kinetic energy theorem) K. This work equals -Ug, the change in the gravitational potential energy of the (earth + object) system. The earth’s kinet ...
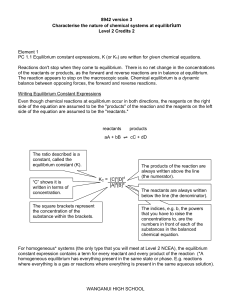
Chemical Equilibrium
... 1 mole contains 6.022 × 1023 molecules/atoms. ◦ This is also known as Avogadro's number. ...
... 1 mole contains 6.022 × 1023 molecules/atoms. ◦ This is also known as Avogadro's number. ...
Wanganui High School
... favours the formation of reactants / shifts equilibrium position to the left o An increase in pressure (volume is reduced) favours the reaction producing the smaller number of moles of gas o A decrease in pressure (volume is increased) favours the reaction producing the larger number of moles of gas ...
... favours the formation of reactants / shifts equilibrium position to the left o An increase in pressure (volume is reduced) favours the reaction producing the smaller number of moles of gas o A decrease in pressure (volume is increased) favours the reaction producing the larger number of moles of gas ...
Chapter 1 Introduction and Definition of Terms
... If body A and B are each in thermal equilibrium with a third body C, they are also in thermal equilibrium with each other. TA = TC and TB = TC => TA = TB The aim of classical thermodynamics is to establish the relationships which exist between equilibrium state of a given system and the influences w ...
... If body A and B are each in thermal equilibrium with a third body C, they are also in thermal equilibrium with each other. TA = TC and TB = TC => TA = TB The aim of classical thermodynamics is to establish the relationships which exist between equilibrium state of a given system and the influences w ...
Kc and Kp Conversions Hess`s Law in Equilibrium Constants
... The equilibrium constant for a net reaction made up of two or more steps can be found from the equilibrium constants for the individual steps.` At 1565 K we have these equilibrium constants: ...
... The equilibrium constant for a net reaction made up of two or more steps can be found from the equilibrium constants for the individual steps.` At 1565 K we have these equilibrium constants: ...
X - Physics
... 1 mole of anything contains 6x1023 particles (Avogadro's number) Even though the force between particles (Newton’s laws) it is impossible to keep track of all 6x1023 particles even with the fastest computer imaginable We must resort to learning about the group properties of all the particles: use th ...
... 1 mole of anything contains 6x1023 particles (Avogadro's number) Even though the force between particles (Newton’s laws) it is impossible to keep track of all 6x1023 particles even with the fastest computer imaginable We must resort to learning about the group properties of all the particles: use th ...
University Studies Chem. 414 Math
... majors areas: thermodynamics, quantum chemistry, kinetics, and statistical mechanics. This course provides an in-depth study of chemical kinetics, and an introduction to quantum chemistry and statistical distributions. The application of quantum mechanics to atomic structure, molecular bonding, and ...
... majors areas: thermodynamics, quantum chemistry, kinetics, and statistical mechanics. This course provides an in-depth study of chemical kinetics, and an introduction to quantum chemistry and statistical distributions. The application of quantum mechanics to atomic structure, molecular bonding, and ...
J.J. Thomson and Duhem`s Lagrangian Approaches to
... questionable alliance between mechanical models and statistical procedures, on the one hand, and the attempt at recasting thermodynamics in accordance with the mathematical structures of Analytical mechanics, on the other. Both research traditions attempted to bridge the gap between the mechanical a ...
... questionable alliance between mechanical models and statistical procedures, on the one hand, and the attempt at recasting thermodynamics in accordance with the mathematical structures of Analytical mechanics, on the other. Both research traditions attempted to bridge the gap between the mechanical a ...
Lecture Outline - Mechanical and Industrial Engineering
... • Is everyone available ? (Midterms) ...
... • Is everyone available ? (Midterms) ...