Atoms and Molecules - Gulfport School District
... • Surrounding the nucleus are electrons which are negatively charged. • Atoms that have equal numbers of electrons and protons are neutrally charged. • Atoms that have gained or lost electrons have charge and are called ions. • Ions are very important human physiology and are involved in the electri ...
... • Surrounding the nucleus are electrons which are negatively charged. • Atoms that have equal numbers of electrons and protons are neutrally charged. • Atoms that have gained or lost electrons have charge and are called ions. • Ions are very important human physiology and are involved in the electri ...
Volatile Organic Compounds
... VOC emissions. Most VOC's contribute to the formation of ground level ozone. Human Health effects ...
... VOC emissions. Most VOC's contribute to the formation of ground level ozone. Human Health effects ...
Chemical Equations
... A compound is broken down into simpler substances (which usually requires energy) Ex: “Powdered ammonium dichromate is strongly heated, producing heat, nitrogen gas, water and chromium(III) oxide.” ...
... A compound is broken down into simpler substances (which usually requires energy) Ex: “Powdered ammonium dichromate is strongly heated, producing heat, nitrogen gas, water and chromium(III) oxide.” ...
Chem 20 Final Exam Outline
... the species involved. Recognize that chemical equations need to be balanced for number of atoms and for charge. Balance chemical equations for number of atoms and for charge. Apply the Law of Conservation of Mass to writing balanced chemical equations. Develop net ionic equations. Recognize that an ...
... the species involved. Recognize that chemical equations need to be balanced for number of atoms and for charge. Balance chemical equations for number of atoms and for charge. Apply the Law of Conservation of Mass to writing balanced chemical equations. Develop net ionic equations. Recognize that an ...
Molecular Biorefining: A New Strategy for Fungible Biofuels
... CHEMICAL ENGINEERING – Molecular Biorefining: A New Strategy for Fungible Biofuels This research focuses on the development of a innovative approach for producing biofuels that exhibit superior cost, carbon, and sustainability characteristics compared to conventional biofuels. We have engineered ind ...
... CHEMICAL ENGINEERING – Molecular Biorefining: A New Strategy for Fungible Biofuels This research focuses on the development of a innovative approach for producing biofuels that exhibit superior cost, carbon, and sustainability characteristics compared to conventional biofuels. We have engineered ind ...
(3.3 × 10!4) + (2.52 × 10!2) = (3.3 × 10!4) × (2.52 × 10!2)
... A pure substance has well defined physical and chemical properties. Pure substances can be classified as elements or compounds. Compounds can be further reduced into two or more elements. Elements consist of only one type of atom. They cannot be decomposed or further simplified by ordinary means. ...
... A pure substance has well defined physical and chemical properties. Pure substances can be classified as elements or compounds. Compounds can be further reduced into two or more elements. Elements consist of only one type of atom. They cannot be decomposed or further simplified by ordinary means. ...
Candle Mass Lab and the Law of Conservation of Matter Notes.
... atomic mass of 12.0 amu and one oxygen atom has an atomic mass of 16.0 amu, what is the molar mass of ...
... atomic mass of 12.0 amu and one oxygen atom has an atomic mass of 16.0 amu, what is the molar mass of ...
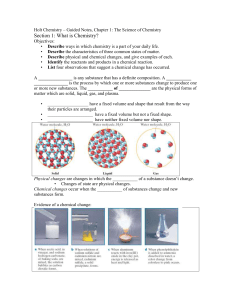
Holt Chemistry – Guided Notes, Chapter 1
... Liquid – particles moving, sliding past each other Gas – constant, random, straight-line motion 6. Give three examples each of physical and chemical changes. Physical – change of state, same substance remains before and after Chemical – one or more new substances forms, chemical reaction ...
... Liquid – particles moving, sliding past each other Gas – constant, random, straight-line motion 6. Give three examples each of physical and chemical changes. Physical – change of state, same substance remains before and after Chemical – one or more new substances forms, chemical reaction ...
Chemistry of Glycolysis
... A) the reduction and phosphorylation of glyceraldehyde 3-phosphate to produce 1,3bisphosphoglycerate. B) the oxidation of a molecule of NAD+ to NADH. C) The reduction of phosphate D) The oxidation of glyceraldehyde and formation of a high energy bond ...
... A) the reduction and phosphorylation of glyceraldehyde 3-phosphate to produce 1,3bisphosphoglycerate. B) the oxidation of a molecule of NAD+ to NADH. C) The reduction of phosphate D) The oxidation of glyceraldehyde and formation of a high energy bond ...
ClickHere - KV HVF , AVADI Chennai
... 13 What are interstitial compounds? Why are such compounds well known for transition metals? 14 Draw a figure to show splitting of degenerate d-orbitals in an octahedral field. How does the magnitude of the ∆o decides the high spin and low spin complexes. 15 The treatment of alkyl chlorides with aqu ...
... 13 What are interstitial compounds? Why are such compounds well known for transition metals? 14 Draw a figure to show splitting of degenerate d-orbitals in an octahedral field. How does the magnitude of the ∆o decides the high spin and low spin complexes. 15 The treatment of alkyl chlorides with aqu ...
MATERIAL FOR GRADE 9 FINAL EXAM 2016
... • Define chemical bond. • Explain why most atoms form chemical bonds. • Differentiate ionic and covalent bonding. • Explain why most chemical bonding is neither purely ionic nor purely covalent. • Classify bonding type according to electronegativity differences. Covalent bonding and molecular compou ...
... • Define chemical bond. • Explain why most atoms form chemical bonds. • Differentiate ionic and covalent bonding. • Explain why most chemical bonding is neither purely ionic nor purely covalent. • Classify bonding type according to electronegativity differences. Covalent bonding and molecular compou ...
chemical reaction?
... • A catalyst is a substance that changes the rate of a chemical reaction without being used up or changed very much. • Catalysts usually ____________ reaction rate by bringing together reactants • _____________ are an example of a catalyst found in living things ...
... • A catalyst is a substance that changes the rate of a chemical reaction without being used up or changed very much. • Catalysts usually ____________ reaction rate by bringing together reactants • _____________ are an example of a catalyst found in living things ...
Chemistry Chapter 2 - Barnstable Academy
... c. They are substances. d. They have properties similar to those of their component elements. ____ 32. Which of the following materials is a substance? a. air c. stainless steel b. gasoline d. silver ____ 33. What is one difference between a mixture and a compound? a. A compound consists of more tha ...
... c. They are substances. d. They have properties similar to those of their component elements. ____ 32. Which of the following materials is a substance? a. air c. stainless steel b. gasoline d. silver ____ 33. What is one difference between a mixture and a compound? a. A compound consists of more tha ...
Notes
... (Don’t worry! There’s an easier way to calculate it without having to memorize the formula!) ...
... (Don’t worry! There’s an easier way to calculate it without having to memorize the formula!) ...
Unit 2: Mixture and Matter Study Guide Ch 2 Vocab to know: Matter
... Chemical property Physical change Chemical change Intensive Homogenous Filtration ...
... Chemical property Physical change Chemical change Intensive Homogenous Filtration ...
PowerPoint for Cornell Notes
... • Neutralization is a type of chemical reaction in which a strong acid and strong base react with each other to form water and salt. Have you ever been unlucky enough to be stung by a wasp or a bee? Bee stings are acidic in nature, which is why a household remedy for a bee sting is baking soda or so ...
... • Neutralization is a type of chemical reaction in which a strong acid and strong base react with each other to form water and salt. Have you ever been unlucky enough to be stung by a wasp or a bee? Bee stings are acidic in nature, which is why a household remedy for a bee sting is baking soda or so ...
Balancing Equations
... When balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. n Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or ...
... When balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. n Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or ...
Unit 10: Chemical Reactions
... Using the equation from question #20, determine how many moles of O2 are needed to completely react with 7.0 moles of C3H8. _____22. Given the number of moles of one of the reactants or products, I can determine the number of moles of another reactant or product that is needed to completely use up t ...
... Using the equation from question #20, determine how many moles of O2 are needed to completely react with 7.0 moles of C3H8. _____22. Given the number of moles of one of the reactants or products, I can determine the number of moles of another reactant or product that is needed to completely use up t ...
processing
... stay ahead of the competition. SwRI chemical engineers have extensive hands-on expertise in pilot plant design, from bench-scale to large demonstration units. Using packed- and fluidized-bed reactors, SwRI develops pilot plants for hydrotreating and hydrocracking hydrocarbons. Batch reactors suppo ...
... stay ahead of the competition. SwRI chemical engineers have extensive hands-on expertise in pilot plant design, from bench-scale to large demonstration units. Using packed- and fluidized-bed reactors, SwRI develops pilot plants for hydrotreating and hydrocracking hydrocarbons. Batch reactors suppo ...
transcript - American Chemical Society
... The product of Dr. Alexander’s research is a device that generates what is essentially an optical fingerprint. Within minutes, it can identify specific bioterrorism threats. In a paper that he published in ACS’ Analytical Chemistry, Dr. Alexander describes experiments in which he used viruses from t ...
... The product of Dr. Alexander’s research is a device that generates what is essentially an optical fingerprint. Within minutes, it can identify specific bioterrorism threats. In a paper that he published in ACS’ Analytical Chemistry, Dr. Alexander describes experiments in which he used viruses from t ...
Use the following to answer questions 1-14:
... ____ 5. Noble gases are very stable; other elements give up, gain, or share electrons to acquire a valence shell like those of noble gases. ____ 6. Ionic bonds form between a metal and a non-metal. ____ 7. Metalloids are located on the left-hand side of the periodic table. ____ 8. Each shell of elec ...
... ____ 5. Noble gases are very stable; other elements give up, gain, or share electrons to acquire a valence shell like those of noble gases. ____ 6. Ionic bonds form between a metal and a non-metal. ____ 7. Metalloids are located on the left-hand side of the periodic table. ____ 8. Each shell of elec ...
Safety Research and Competitiveness and First
... like China (where demand is growing) and the USA and the Middle East (where energy and raw materials are cheap) has put the EU industry under increasing pressure from international competition. If this loss of competitiveness is not addressed the EU risks becoming an increasingly high cost region wi ...
... like China (where demand is growing) and the USA and the Middle East (where energy and raw materials are cheap) has put the EU industry under increasing pressure from international competition. If this loss of competitiveness is not addressed the EU risks becoming an increasingly high cost region wi ...
Section 8-4
... – Made of C, H, O, P, N, and S – Used to build and repair body parts – Monomers are amino acids • There are 20 amino acids in all living things ...
... – Made of C, H, O, P, N, and S – Used to build and repair body parts – Monomers are amino acids • There are 20 amino acids in all living things ...
Chemical Names and Formulas
... Goal Practise naming and writing formulas for different substances. What to Do Complete the following table. Chemical formula ...
... Goal Practise naming and writing formulas for different substances. What to Do Complete the following table. Chemical formula ...
VX (nerve agent)

VX (IUPAC name O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate) is an extremely toxic substance that has no known uses except in chemical warfare as a nerve agent. It is a tasteless and odorless liquid. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations in UN Resolution 687. The production and stockpiling of VX exceeding 100 grams per year was outlawed by the Chemical Weapons Convention of 1993.The VX nerve agent is the best-known of the V-series of nerve agents and is considered an area denial weapon due to its physical properties.