06_reactions
... CH2O + Ag(NH3)2+ + H2O → CHOOH + Ag + 2NH3 + 2H+ Since an obvious silver ‘mirror’ forms on the flask if a reaction occurs, this is commonly used to test whether a substance is a ketone or an aldehyde. A ketone cannot be oxidised, so no silver mirror would form. Tollens’ reagent is ammoniacal silver ...
... CH2O + Ag(NH3)2+ + H2O → CHOOH + Ag + 2NH3 + 2H+ Since an obvious silver ‘mirror’ forms on the flask if a reaction occurs, this is commonly used to test whether a substance is a ketone or an aldehyde. A ketone cannot be oxidised, so no silver mirror would form. Tollens’ reagent is ammoniacal silver ...
Chemical bonding
... • A physical change does not change the substance • A chemical change (AKA chemical reaction) does change the substance • Chemical changes are accompanied by physical changes ...
... • A physical change does not change the substance • A chemical change (AKA chemical reaction) does change the substance • Chemical changes are accompanied by physical changes ...
Master Sheet Mole:Mole Ratios and Mass
... order to make the drug that you are in charge of. How will you do this? You will use, in part, mole to mole ratios. How to use mole: mole ratios to determine how much of a chemical is needed for a reaction: 1. Balance the chemical equation. 2. Use factor label! 3. Convert to moles (using atomic or m ...
... order to make the drug that you are in charge of. How will you do this? You will use, in part, mole to mole ratios. How to use mole: mole ratios to determine how much of a chemical is needed for a reaction: 1. Balance the chemical equation. 2. Use factor label! 3. Convert to moles (using atomic or m ...
CHEMICAL AND BIOCHEMICAL TOXICOLOGY INTRODUCTION
... by which agent X could have produced effect Y?) 7. Experimental evidence (toxicological experiments, often in model systems, to demonstrate the toxic effect and to support the proposed mechanism) ...
... by which agent X could have produced effect Y?) 7. Experimental evidence (toxicological experiments, often in model systems, to demonstrate the toxic effect and to support the proposed mechanism) ...
physical change
... Example 1: Hydrogen, Oxygen and Nitrogen are colorless gases but they combine with carbon to form nylon, a flexible solid. ...
... Example 1: Hydrogen, Oxygen and Nitrogen are colorless gases but they combine with carbon to form nylon, a flexible solid. ...
Carbonyl Compounds Prior Knowledge
... be able to apply IUPAC rules for nomenclature to alcohols, aldehydes, ketones and carboxylic acids limited to chains with up to 6 carbon atoms understand that alcohols can be classified as primary, secondary or tertiary understand that tertiary alcohols are not easily oxidised understand that primar ...
... be able to apply IUPAC rules for nomenclature to alcohols, aldehydes, ketones and carboxylic acids limited to chains with up to 6 carbon atoms understand that alcohols can be classified as primary, secondary or tertiary understand that tertiary alcohols are not easily oxidised understand that primar ...
Antimicrobial Agents Suitable for Use in Lab. Animal Facility
... From AALAS Reference Directory 2007 ...
... From AALAS Reference Directory 2007 ...
3.1 - Weathering Define mechanical and chemical weathering
... Many limestone caves have been formed by the action of carbonic acid. ...
... Many limestone caves have been formed by the action of carbonic acid. ...
Answer key
... blue color density flammability (burns) solubility (dissolves) reacts with acid supports combustion sour taste ...
... blue color density flammability (burns) solubility (dissolves) reacts with acid supports combustion sour taste ...
Chapter 1 Matter and Change
... Mixtures are a physical blend of at least two substances; have variable composition. They can be either: 1) Heterogeneous – the mixture is not uniform in composition • Chocolate chip cookie, gravel, soil. 2) Homogeneous - same composition throughout; called “solutions” • Kool-aid, air, salt water ...
... Mixtures are a physical blend of at least two substances; have variable composition. They can be either: 1) Heterogeneous – the mixture is not uniform in composition • Chocolate chip cookie, gravel, soil. 2) Homogeneous - same composition throughout; called “solutions” • Kool-aid, air, salt water ...
Quick Breads - pkwy.k12.mo.us
... the baking soda comes into contact with the acid, the chemical reaction occurs regardless if heat is present or not ...
... the baking soda comes into contact with the acid, the chemical reaction occurs regardless if heat is present or not ...
Chemical Technology - Engineers Institute of India
... 2. The liquid droplets of FeS are caught in the molten horizontal batch and silica gangue is trapped and fluxed with lime, floating on the top of the molten FeS matte. The liquid FeS is tapped periodically and granulated in water to produce 4 mm grains for further roasting operation. 3. Hot gases at ...
... 2. The liquid droplets of FeS are caught in the molten horizontal batch and silica gangue is trapped and fluxed with lime, floating on the top of the molten FeS matte. The liquid FeS is tapped periodically and granulated in water to produce 4 mm grains for further roasting operation. 3. Hot gases at ...
Why Study Chemistry
... – how hot or cold something is (a physical property) – related to the average (kinetic) energy of the substance (not the total energy) – Measured in units of • Degrees Fahrenheit (oF) • Degrees Celsius (oC) ...
... – how hot or cold something is (a physical property) – related to the average (kinetic) energy of the substance (not the total energy) – Measured in units of • Degrees Fahrenheit (oF) • Degrees Celsius (oC) ...
introduction to matter
... added together which results in a simple mixing without reaction. Chemical changes are changes that produce new substances. These are basically chemical reactions and can be indicated by: (1) release of gas, (2) permanent color change or (3) formation of an insoluble solid (called precipitate) upon ...
... added together which results in a simple mixing without reaction. Chemical changes are changes that produce new substances. These are basically chemical reactions and can be indicated by: (1) release of gas, (2) permanent color change or (3) formation of an insoluble solid (called precipitate) upon ...
Utah - Wavefunction, Inc.
... matter how they are rearranged; the total mass stays the same. Although energy can be absorbed or released in a chemical reaction, the total amount of energy and matter in it remains constant. Many reactions attain a state of equilibrium. Many ordinary activities, such as baking, involve chemical re ...
... matter how they are rearranged; the total mass stays the same. Although energy can be absorbed or released in a chemical reaction, the total amount of energy and matter in it remains constant. Many reactions attain a state of equilibrium. Many ordinary activities, such as baking, involve chemical re ...
Lesson 1 of 6
... • In any chemical reaction, mass is conserved. – In other words, the mass of the reactant(s) is the same as the mass of the product(s). – The elements on one side of the equation are the same as those on the other. – Matter cannot be created nor destroyed. ...
... • In any chemical reaction, mass is conserved. – In other words, the mass of the reactant(s) is the same as the mass of the product(s). – The elements on one side of the equation are the same as those on the other. – Matter cannot be created nor destroyed. ...
chemical reaction
... Factors Affecting Rates of Reactions, continued • Concentration In general, a high concentration of reactants causes a fast rate of reaction. Concentration is a measure of the amount of one substance when it is dissolved in another substance. • When concentration is high, there are many reactant par ...
... Factors Affecting Rates of Reactions, continued • Concentration In general, a high concentration of reactants causes a fast rate of reaction. Concentration is a measure of the amount of one substance when it is dissolved in another substance. • When concentration is high, there are many reactant par ...
practice final examination
... 10. Answer true or false for each of the following questions below (circle your choice): a) ...
... 10. Answer true or false for each of the following questions below (circle your choice): a) ...
4 New Online Chemistry References - Available Now!
... 4 New Online Chemistry References - Available Now! The library is excited to announce access to four new online chemistry references. These new references give students and faculty the ability to quickly locate key information about the properties of chemical compounds. You may access these resource ...
... 4 New Online Chemistry References - Available Now! The library is excited to announce access to four new online chemistry references. These new references give students and faculty the ability to quickly locate key information about the properties of chemical compounds. You may access these resource ...
CHEM MINI-COURSE SERIES M1.2___
... chemical equation must show the fact that no atom can be destroyed or created; i.e., the same number of each type of atoms must appear on both sides of an equation. The atoms merely rearrange or regroup into different elements or compounds; they will not change into other atoms or be lost through a ...
... chemical equation must show the fact that no atom can be destroyed or created; i.e., the same number of each type of atoms must appear on both sides of an equation. The atoms merely rearrange or regroup into different elements or compounds; they will not change into other atoms or be lost through a ...
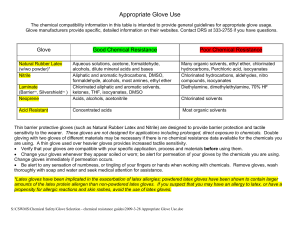
Appropriate Glove Use
... Thin barrier protective gloves (such as Natural Rubber Latex and Nitrile) are designed to provide barrier protection and tactile sensitivity to the wearer. These gloves are not designed for applications including prolonged, direct exposure to chemicals. Double gloving with two gloves of different ma ...
... Thin barrier protective gloves (such as Natural Rubber Latex and Nitrile) are designed to provide barrier protection and tactile sensitivity to the wearer. These gloves are not designed for applications including prolonged, direct exposure to chemicals. Double gloving with two gloves of different ma ...
1-BUTANESULFONIC ACID SODIUM SALT
... DUDLEY CORPORATION provides the information herein in good faith but makes no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the ...
... DUDLEY CORPORATION provides the information herein in good faith but makes no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the ...
Chapter 4 - WordPress.com
... Types of Reactions • 4 different types of Reactions: – Combination: A + B AB – Decomposition: AB A + B – Single Replacement: A + BC AC + B ...
... Types of Reactions • 4 different types of Reactions: – Combination: A + B AB – Decomposition: AB A + B – Single Replacement: A + BC AC + B ...
Document
... in location of the phenyl group. Isoflavones are produced via a branch of the general phenylpropanoid pathway that produces flavonoid compounds in higher plants. Soybeans are the most common source of isoflavones in human food; the major isoflavones in soybean are genistein and daidzein. The phenylp ...
... in location of the phenyl group. Isoflavones are produced via a branch of the general phenylpropanoid pathway that produces flavonoid compounds in higher plants. Soybeans are the most common source of isoflavones in human food; the major isoflavones in soybean are genistein and daidzein. The phenylp ...
VX (nerve agent)

VX (IUPAC name O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate) is an extremely toxic substance that has no known uses except in chemical warfare as a nerve agent. It is a tasteless and odorless liquid. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations in UN Resolution 687. The production and stockpiling of VX exceeding 100 grams per year was outlawed by the Chemical Weapons Convention of 1993.The VX nerve agent is the best-known of the V-series of nerve agents and is considered an area denial weapon due to its physical properties.