Chapter 6 Electronic Structure of Atoms
... • For a one-electron hydrogen atom, orbitals on the same energy level have the same energy. • That is, they are degenerate. Electronic Structure of Atoms © 2009, Prentice-Hall, Inc. ...
... • For a one-electron hydrogen atom, orbitals on the same energy level have the same energy. • That is, they are degenerate. Electronic Structure of Atoms © 2009, Prentice-Hall, Inc. ...
High Performance Multi Barrier Thermionic Devices
... (3D) states with energies above the barrier. Superlattice structure chosen here has wide wells with several quantized states that reduce the effect of 3D states on thermionic current. A more important assumption is that the lateral momentum of electrons is assumed to be conserved. As we see in the p ...
... (3D) states with energies above the barrier. Superlattice structure chosen here has wide wells with several quantized states that reduce the effect of 3D states on thermionic current. A more important assumption is that the lateral momentum of electrons is assumed to be conserved. As we see in the p ...
S P E C T R O S C O... F E R R O I C S Y S...
... complicated to measure both magnetic and electric properties simultaneously. This creates a need for an intermediate method that offers sub-micron lateral resolution and short measurement times, yet produces enough information about all ferroic properties of a system. To address this issue we develo ...
... complicated to measure both magnetic and electric properties simultaneously. This creates a need for an intermediate method that offers sub-micron lateral resolution and short measurement times, yet produces enough information about all ferroic properties of a system. To address this issue we develo ...
Chapter 6 Electronic Structure of Atoms
... • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms ...
... • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level are no longer degenerate. Electronic Structure of Atoms ...
Scanning-probe spectroscopy of semiconductor donor molecules LETTERS
... V exc = 15 mV r.m.s. The local measurements consistently showed three broad peaks labelled A, B and C. b, Capacitance curves acquired at the same position as in a, but over the indicated expanded voltage range. To investigate the structure in detail, here we used a smaller excitation amplitude of 3. ...
... V exc = 15 mV r.m.s. The local measurements consistently showed three broad peaks labelled A, B and C. b, Capacitance curves acquired at the same position as in a, but over the indicated expanded voltage range. To investigate the structure in detail, here we used a smaller excitation amplitude of 3. ...
Introduction to the Bethe Ansatz II
... work on quantum spin chains from the early sixties until the present. Some of the advances achieved via the Bethe ansatz emerged in direct response to experimental data which had remained unexplained by standard approximations used in many-body theory. In Part I of this series1 we introduced the Bet ...
... work on quantum spin chains from the early sixties until the present. Some of the advances achieved via the Bethe ansatz emerged in direct response to experimental data which had remained unexplained by standard approximations used in many-body theory. In Part I of this series1 we introduced the Bet ...
in my paper on Period 4
... neutrons. I have drawn the neutrons smaller and as circles only as a convenience—to separate them from the protons at a glance, and to fit them into already crowded diagrams. However, I need to include the neutrons to explain the densities in Period 4, as you will now see. Current theory thinks Iron ...
... neutrons. I have drawn the neutrons smaller and as circles only as a convenience—to separate them from the protons at a glance, and to fit them into already crowded diagrams. However, I need to include the neutrons to explain the densities in Period 4, as you will now see. Current theory thinks Iron ...
Part 2: Two Examples of the Boltzmann Distribution
... We will look in particular at properties that can be measured in the laboratory, such as the specific heat capacity and its variation with temperature. ...
... We will look in particular at properties that can be measured in the laboratory, such as the specific heat capacity and its variation with temperature. ...
Document
... Example 8.10 Alkali Metal and Halogen Reactions Write a balanced chemical equation for each reaction. a. the reaction between potassium metal and bromine gas b. the reaction between rubidium metal and liquid water c. the reaction between gaseous chlorine and solid iodine ...
... Example 8.10 Alkali Metal and Halogen Reactions Write a balanced chemical equation for each reaction. a. the reaction between potassium metal and bromine gas b. the reaction between rubidium metal and liquid water c. the reaction between gaseous chlorine and solid iodine ...
Introduction - LPPD - University of Illinois at Chicago
... and paramagnets, ferromagnets will retain magnetic properties in the absence of a magnetic field. Another classification of magnetism is called superparamagnetism and occurs only at the nanoscale. Ferromagnetic particles with diameters less than 14 nm, lose their ferromagnetic properties and behave ...
... and paramagnets, ferromagnets will retain magnetic properties in the absence of a magnetic field. Another classification of magnetism is called superparamagnetism and occurs only at the nanoscale. Ferromagnetic particles with diameters less than 14 nm, lose their ferromagnetic properties and behave ...
$doc.title
... Spectroscopy comes from the Latin “spectron” for spirit or ghost and the Greek “σκοπιεν” for to see. These roots are very telling, because in molecular spectroscopy you use light to interrogate matter, but you actually never see the molecules, only their influence on the light. Different spectroscop ...
... Spectroscopy comes from the Latin “spectron” for spirit or ghost and the Greek “σκοπιεν” for to see. These roots are very telling, because in molecular spectroscopy you use light to interrogate matter, but you actually never see the molecules, only their influence on the light. Different spectroscop ...
NMR SPectroscopy
... The magnetic effects of nuclei in close proximity to those being observed have an effect on the local magnetic field, and therefore DE Specifically, when proton is close enough to another proton, typically by being on an adjacent carbon (vicinal), it can “feel” the magnetic effects generated by that ...
... The magnetic effects of nuclei in close proximity to those being observed have an effect on the local magnetic field, and therefore DE Specifically, when proton is close enough to another proton, typically by being on an adjacent carbon (vicinal), it can “feel” the magnetic effects generated by that ...
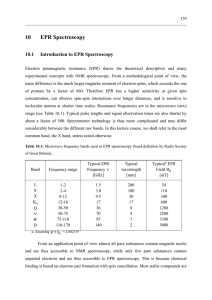
10 EPR Spectroscopy
... concentration regime where interactions between paramagnetic centers are negligible. An exception are systems where the distance between two (or more) paramagnetic centers is relatively well defined and is measured by EPR (Section 10.3.6). An isolated paramagnetic center can contain a single unpaire ...
... concentration regime where interactions between paramagnetic centers are negligible. An exception are systems where the distance between two (or more) paramagnetic centers is relatively well defined and is measured by EPR (Section 10.3.6). An isolated paramagnetic center can contain a single unpaire ...
Quantum rings for beginners: energy spectra and persistent currents
... stress that most of the phenomena shown have been published before (in many case by several authors) and we will give reference to earlier work. We take an approach where we analyse the many-body excitation spectrum and its relation to the single particle spectrum and electron localization along the ...
... stress that most of the phenomena shown have been published before (in many case by several authors) and we will give reference to earlier work. We take an approach where we analyse the many-body excitation spectrum and its relation to the single particle spectrum and electron localization along the ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.