Electrons BellwoodNotes
... “building up” An electron occupies the lowest energy possible The levels follow a pattern of increasing energy Fill starting at nucleus (Bohr Models!) p subshell 3 orbitals ...
... “building up” An electron occupies the lowest energy possible The levels follow a pattern of increasing energy Fill starting at nucleus (Bohr Models!) p subshell 3 orbitals ...
NMR_1
... a characteristic frequency, To observe resonance, all we have to do is irradiate them with electromagnetic radiation of the appropriate frequency. •It’s easy to understand that different nucleus “type” will give different NMR signal. (remember v =w/2= B0/2 ? Thus, different cause different v !! ...
... a characteristic frequency, To observe resonance, all we have to do is irradiate them with electromagnetic radiation of the appropriate frequency. •It’s easy to understand that different nucleus “type” will give different NMR signal. (remember v =w/2= B0/2 ? Thus, different cause different v !! ...
Surface contribution to giant magnetoresistance in Fe/Cr/Fe films K. W
... into the effective field generated by the interaction between the layered systems. In order to calculate the global value for the conductivity, we sum up all the planar contributions in the case of current in plane (CIP) geometry while the case of current perpendicular to plane (CPP) geometry requir ...
... into the effective field generated by the interaction between the layered systems. In order to calculate the global value for the conductivity, we sum up all the planar contributions in the case of current in plane (CIP) geometry while the case of current perpendicular to plane (CPP) geometry requir ...
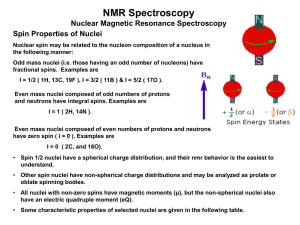
for I = 1/2 nuclei - Instrumentation Engineer`s Site
... • Although NMR spectra could be, and have been, obtained using a fixed magnetic field and sweeping the frequency of the electromagnetic radiation, this more typically involved using a fixed frequency source and varying the current (and hence magnetic field) in an electromagnet to observe the resonan ...
... • Although NMR spectra could be, and have been, obtained using a fixed magnetic field and sweeping the frequency of the electromagnetic radiation, this more typically involved using a fixed frequency source and varying the current (and hence magnetic field) in an electromagnet to observe the resonan ...
Chapter 5
... the spin quantum number (ms). E. none of these choices is correct 21. Atomic orbitals developed using quantum mechanics A. describe regions of space in which one is most likely to find an electron. B. describe exact paths for electron motion. C. give a description of the atomic structure which is es ...
... the spin quantum number (ms). E. none of these choices is correct 21. Atomic orbitals developed using quantum mechanics A. describe regions of space in which one is most likely to find an electron. B. describe exact paths for electron motion. C. give a description of the atomic structure which is es ...
Subnanometre resolution in three-dimensional magnetic resonance
... spin-state dependent fluorescence17. To image the three-dimensional distribution of dark spins via NV-MRI, we apply a local magnetic-field gradient with a scanning magnetic tip. The magnetic tip provides a narrow spatial volume (a ‘resonant slice’) in which dark spins are on resonance with a driving r ...
... spin-state dependent fluorescence17. To image the three-dimensional distribution of dark spins via NV-MRI, we apply a local magnetic-field gradient with a scanning magnetic tip. The magnetic tip provides a narrow spatial volume (a ‘resonant slice’) in which dark spins are on resonance with a driving r ...
physics colloquium
... Niels Bohr Institute, University of Copenhagen, DK-2100 Copenhagen, Denmark / Department of Physics, Binghamton University ...
... Niels Bohr Institute, University of Copenhagen, DK-2100 Copenhagen, Denmark / Department of Physics, Binghamton University ...
Spin and orbital Kondo effect in electrostatically coupled quantum dots S. L
... transparency region (VSD, h ≈ 0) corresponds to the spin Kondo effect at the dots (εi+ = εi–, 2*SU(2)). The enhanced conductance in this region, marked by the dark circle, is due to the orbital Kondo effect (ε1+ = ε2– for g1 = g2, or ε1+ = ε2+ for g1 = –g2). The orbital degeneracy for the same spin ...
... transparency region (VSD, h ≈ 0) corresponds to the spin Kondo effect at the dots (εi+ = εi–, 2*SU(2)). The enhanced conductance in this region, marked by the dark circle, is due to the orbital Kondo effect (ε1+ = ε2– for g1 = g2, or ε1+ = ε2+ for g1 = –g2). The orbital degeneracy for the same spin ...
Chapter 2 Second Quantisation - Theory of Condensed Matter
... it. Next define F0 to be the space generated by |⌦i. We may then introduce a set of states |ki ⌘ a†k |⌦i, k = 0, 2⇡/L, . . . by applying oscillator creation operators to the vacuum. Physically, the state |ki has the significance of a single harmonic oscillator quantum excited in mode k. In other wor ...
... it. Next define F0 to be the space generated by |⌦i. We may then introduce a set of states |ki ⌘ a†k |⌦i, k = 0, 2⇡/L, . . . by applying oscillator creation operators to the vacuum. Physically, the state |ki has the significance of a single harmonic oscillator quantum excited in mode k. In other wor ...
Orbitals and energy levels
... anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller. ...
... anywhere in the blurry region, but you cannot tell its location at any instant. The electron cloud of an atom can be compared to a spinning airplane propeller. ...
Molekylfysik - Leiden Univ
... The gas inside the tube; that emits blue light is mercury, but the same can be done with H2. The gas pressure in the tubes are low, because of natural radioactivity and cosmic rays there are always few free electrons and ions. When something around 2000-3000 volts is applied to these tubes under low ...
... The gas inside the tube; that emits blue light is mercury, but the same can be done with H2. The gas pressure in the tubes are low, because of natural radioactivity and cosmic rays there are always few free electrons and ions. When something around 2000-3000 volts is applied to these tubes under low ...
p-shell hybridization and Hund`s-rule mitigation
... with the sum rule L z P(L z ) = 1 conserving probability. Our calculations of four interacting electrons in an anisotropic quantum dot, (5), and (4), proceed through two successive exact-diagonalization procedures. In the first step, we compute the energy levels and eigenstates of four interacting e ...
... with the sum rule L z P(L z ) = 1 conserving probability. Our calculations of four interacting electrons in an anisotropic quantum dot, (5), and (4), proceed through two successive exact-diagonalization procedures. In the first step, we compute the energy levels and eigenstates of four interacting e ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.