purdue university - IUPUI ScholarWorks
... I certify that in the preparation of this thesis, I have observed the provisions of Purdue University Teaching, Research, and Outreach Policy on Research Misconduct (VIII.3.1), October 1, 2008.* Further, I certify that this work is free of plagiarism and all materials appearing in this thesis/disser ...
... I certify that in the preparation of this thesis, I have observed the provisions of Purdue University Teaching, Research, and Outreach Policy on Research Misconduct (VIII.3.1), October 1, 2008.* Further, I certify that this work is free of plagiarism and all materials appearing in this thesis/disser ...
Sample pages 2 PDF
... distribution while all particles grow and no additional nucleation appears. This selfregulating mechanism of size distribution is often called “focusing effect” [29]. On the whole, particle growth is controlled by competition of two processes: a decrease in bulk energy, which advances growth (absorp ...
... distribution while all particles grow and no additional nucleation appears. This selfregulating mechanism of size distribution is often called “focusing effect” [29]. On the whole, particle growth is controlled by competition of two processes: a decrease in bulk energy, which advances growth (absorp ...
Synthesis and Characterization of Amorphous and Hybrid Materials
... where R is an alkyl group, CxH2x+1. The hydrolysis reaction (eq. 1) replaces alkoxide group (OR) with hydroxyl group (OH). Subsequent condensation reactions involving the silanol group produce siloxane bonds (Si-O-Si) and the by-products alcohol (ROH) (eq. 2) or water (eq. 3). Under most conditions, ...
... where R is an alkyl group, CxH2x+1. The hydrolysis reaction (eq. 1) replaces alkoxide group (OR) with hydroxyl group (OH). Subsequent condensation reactions involving the silanol group produce siloxane bonds (Si-O-Si) and the by-products alcohol (ROH) (eq. 2) or water (eq. 3). Under most conditions, ...
Multiple-choice questions : 1. The following graph shows the volume
... 14. Consider the reaction of excess dilute hydrochloric acid and magnesium ribbon, which of the following parameters is/are NOT changed upon changes in the concentration of the hydrochloric acid, given other factors are kept constant? (1) The total heat energy released from the reaction mixture (2) ...
... 14. Consider the reaction of excess dilute hydrochloric acid and magnesium ribbon, which of the following parameters is/are NOT changed upon changes in the concentration of the hydrochloric acid, given other factors are kept constant? (1) The total heat energy released from the reaction mixture (2) ...
Supplemental Notes
... 1. In terms of mass: The mass of one mole of any substance is equal to its relative atomic or molecular mass taken in grams, e.g., 1 mole of Fe = 56 g (Atomic mass, Ar) 1 mole of H2O = 18 g (Molecular mass, Mr) 2. In terms of number: One mole of any substance contains particles equal to 6.02 x 1023. ...
... 1. In terms of mass: The mass of one mole of any substance is equal to its relative atomic or molecular mass taken in grams, e.g., 1 mole of Fe = 56 g (Atomic mass, Ar) 1 mole of H2O = 18 g (Molecular mass, Mr) 2. In terms of number: One mole of any substance contains particles equal to 6.02 x 1023. ...
analytical applications of surface-modified fused silica capillaries
... Doctor of Philosophy in Chemistry The University of Montana ...
... Doctor of Philosophy in Chemistry The University of Montana ...
Full-Text PDF
... and mesopores have been synthesized by delaminating the layered zeolite precursors MCM-22 and ferrierite [14]. However, none of these techniques yields a regular distribution of mesopores, let alone an ideal channel system of mesopores structurally connected with the regular micropores of the zeolit ...
... and mesopores have been synthesized by delaminating the layered zeolite precursors MCM-22 and ferrierite [14]. However, none of these techniques yields a regular distribution of mesopores, let alone an ideal channel system of mesopores structurally connected with the regular micropores of the zeolit ...
JOURNAL OF FLOW CHEMISTRY (ISSN: 2062
... enantiomerically pure compounds, the armoury of novel stereoselective synthetic methods is continuously expanding. The use of biocatalysts for these purposes grows with this expansion16-19 since biocatalysis often requires milder conditions in stereoselective reaction than chemocatalysis. Hydrolases ...
... enantiomerically pure compounds, the armoury of novel stereoselective synthetic methods is continuously expanding. The use of biocatalysts for these purposes grows with this expansion16-19 since biocatalysis often requires milder conditions in stereoselective reaction than chemocatalysis. Hydrolases ...
A Review of using Spray Pyrolysis through Sol-gel
... a variety of glasses, ceramics, inorganic fillers and coatings.12 In some industrial applications, the coatings were used for chemical protection (such as corrosion inhibitors) and mechanical protection (such as abrasion resistance).14 The technology was also used in biomedical applications (such as ...
... a variety of glasses, ceramics, inorganic fillers and coatings.12 In some industrial applications, the coatings were used for chemical protection (such as corrosion inhibitors) and mechanical protection (such as abrasion resistance).14 The technology was also used in biomedical applications (such as ...
24. The following reaction is at equilibrium
... 22. The following statements refer to a mixture (reaction quotient Q) that is prepared and then allowed to come to equilibrium. Which statement is NOT CORRECT? (A) A reaction will proceed to make the value of Q approach that of K. (B) If Q = K there is no change. (C) If Q > K, the reaction goes to ...
... 22. The following statements refer to a mixture (reaction quotient Q) that is prepared and then allowed to come to equilibrium. Which statement is NOT CORRECT? (A) A reaction will proceed to make the value of Q approach that of K. (B) If Q = K there is no change. (C) If Q > K, the reaction goes to ...
Kinetics and Equilibrium
... solids and liquids in calculating Keq. Why? *If temperature is constant, then partial pressure of a gas directly related to the concentration (mol/L) ...
... solids and liquids in calculating Keq. Why? *If temperature is constant, then partial pressure of a gas directly related to the concentration (mol/L) ...
How do we distinguish substances?
... If you think about it, the fact that we can now detect or identify all of the substances present in a given system is an incredible achievement of human kind. Most of the systems we deal with, natural or artificial, are mixtures of many different substances. Many of these mixtures are homogeneous: c ...
... If you think about it, the fact that we can now detect or identify all of the substances present in a given system is an incredible achievement of human kind. Most of the systems we deal with, natural or artificial, are mixtures of many different substances. Many of these mixtures are homogeneous: c ...
Separation of 2 (3), 9 (10), 16 (17), 23 (24)
... group induces a steric effect. With this phase (31), for the first time it is possible to separate practically all four isomers of 12, as shown in Figure 3. The separation of the nickel phthalocyanines 3-11 using phase 31 is less successful: although a complete separation of the C2V and Cs isomers o ...
... group induces a steric effect. With this phase (31), for the first time it is possible to separate practically all four isomers of 12, as shown in Figure 3. The separation of the nickel phthalocyanines 3-11 using phase 31 is less successful: although a complete separation of the C2V and Cs isomers o ...
Measuring Rates
... result with that for a first order process. Discuss differences and similarities between the two expressions and evaluate the extent to which t1/2 for a second order reaction may be a useful quantity in making judgments about kinetic stability. ...
... result with that for a first order process. Discuss differences and similarities between the two expressions and evaluate the extent to which t1/2 for a second order reaction may be a useful quantity in making judgments about kinetic stability. ...
Bulgarian Chemical Communications, Volume 41, Number 4 (pp
... The degradation of diazo dyes Brilliant Yellow (BY) and Bismark Brown (BB) was investigated by the photoFenton-like process Fe2+/ammonium persulphate (APS)/UV in acidic pH medium. The influence of various reaction parameters like pH, concentration of Fe2+ ions/APS, structure of the dye and effect of ...
... The degradation of diazo dyes Brilliant Yellow (BY) and Bismark Brown (BB) was investigated by the photoFenton-like process Fe2+/ammonium persulphate (APS)/UV in acidic pH medium. The influence of various reaction parameters like pH, concentration of Fe2+ ions/APS, structure of the dye and effect of ...
full text pdf
... provided a product with a narrow particle size distribution. The 160 UPZ mill from Hosokawa-Alpine was used for impact milling. Milling of the material was due to impact of the steel tines spaced on the shield and on the spinning rotor. The milling was conducted at rotor speed of 11 400 rpm and with ...
... provided a product with a narrow particle size distribution. The 160 UPZ mill from Hosokawa-Alpine was used for impact milling. Milling of the material was due to impact of the steel tines spaced on the shield and on the spinning rotor. The milling was conducted at rotor speed of 11 400 rpm and with ...
Modeling the Rate of Heterogeneous Reactions
... Lateral interactions can be incorporated into this abstract view as well. From the modeling point of view one distinguishes between hard sphere and soft interactions. Hard sphere interactions are very strong lateral interactions, in which the adsorbed species behave as hard spheres and exclude neigh ...
... Lateral interactions can be incorporated into this abstract view as well. From the modeling point of view one distinguishes between hard sphere and soft interactions. Hard sphere interactions are very strong lateral interactions, in which the adsorbed species behave as hard spheres and exclude neigh ...
Preparation and Properties of an Aqueous Ferrofluid
... magnetic stirring bar that remains in the filtration flask should be covered with a black sludge, which may or may not exhibit spikes at the ends of the magnet. Gently pour the stirring bar and attached sludge into a plastic weighing boat. Remember that ferrofluids are messy and can easily and perma ...
... magnetic stirring bar that remains in the filtration flask should be covered with a black sludge, which may or may not exhibit spikes at the ends of the magnet. Gently pour the stirring bar and attached sludge into a plastic weighing boat. Remember that ferrofluids are messy and can easily and perma ...
Equilibrium
... for a given mass of particles. An increase in surface area increases the amount of the reactant exposed for reaction, which increases the collision frequency and the reaction rate. One way to increase the surface area of solid reactants is to dissolve them. In solution, particles are separated and m ...
... for a given mass of particles. An increase in surface area increases the amount of the reactant exposed for reaction, which increases the collision frequency and the reaction rate. One way to increase the surface area of solid reactants is to dissolve them. In solution, particles are separated and m ...
Unit- 5.pmd
... or residual attractive forces. These forces of the adsorbent are responsible for attracting the adsorbate particles on its surface.The extent of adsorption increases with the increase of surface area per unit mass of the adsorbent at a given temperature and pressure. Another important factor featuri ...
... or residual attractive forces. These forces of the adsorbent are responsible for attracting the adsorbate particles on its surface.The extent of adsorption increases with the increase of surface area per unit mass of the adsorbent at a given temperature and pressure. Another important factor featuri ...
Noninteracting Particle Systems - Particle Solids Interactions group
... with a heat bath at temperature T , it is more natural and convenient to treat the ideal gas in the canonical ensemble. However, because the particles are not localized, they cannot be distinguished from each other as were the harmonic oscillators considered in Example 4.4 and the spins in Chapter 5 ...
... with a heat bath at temperature T , it is more natural and convenient to treat the ideal gas in the canonical ensemble. However, because the particles are not localized, they cannot be distinguished from each other as were the harmonic oscillators considered in Example 4.4 and the spins in Chapter 5 ...
Surface chemistry and Catalysis
... layer adsorption. In BET it is assumed that the solid surface possesses uniform, localized sites and adsorption at one site does not affect adsorption at neighboring sites . It is further assumed that the molecule can be adsorbed in second, third…and nth layer, the surface area available for the nth ...
... layer adsorption. In BET it is assumed that the solid surface possesses uniform, localized sites and adsorption at one site does not affect adsorption at neighboring sites . It is further assumed that the molecule can be adsorbed in second, third…and nth layer, the surface area available for the nth ...
Energy (eV) - Integrated Composites Lab
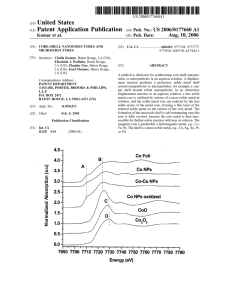
... [0003] Iron-group nanoparticles, i.e., nanoparticles of cobalt, iron, and nickel, have unusual and useful magnetic properties. For example, their coercivity is enhanced as compared to thin ?lms or microscale particles, making them useful in high-density data storage, due to their inherent high ...
... [0003] Iron-group nanoparticles, i.e., nanoparticles of cobalt, iron, and nickel, have unusual and useful magnetic properties. For example, their coercivity is enhanced as compared to thin ?lms or microscale particles, making them useful in high-density data storage, due to their inherent high ...
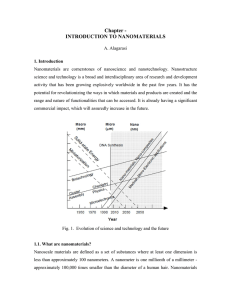
Chapter - INTRODUCTION TO NANOMATERIALS
... is controlling the growth of the particles and then stopping the newly formed particles from agglomerating. Other technical issues are ensuring the reactions are complete so that no unwanted reactant is left on the product and completely removing any growth aids that ...
... is controlling the growth of the particles and then stopping the newly formed particles from agglomerating. Other technical issues are ensuring the reactions are complete so that no unwanted reactant is left on the product and completely removing any growth aids that ...
The effect of confinement on chemical reactions
... The results in Fig. 4 show that there is a significant enhancement of the equilibrium yield for the confined fluid, as the mole fraction of the ester is more than doubled in the pore phase. This difference was shown [5] to be due to the selective adsorption of the ester, which has a more favorable f ...
... The results in Fig. 4 show that there is a significant enhancement of the equilibrium yield for the confined fluid, as the mole fraction of the ester is more than doubled in the pore phase. This difference was shown [5] to be due to the selective adsorption of the ester, which has a more favorable f ...