Thermochemistry
... U = U products U reactants and H = H products H reactants. One thing is sometimes not made very clear. "Reactants" and "products" in these equations means that the reactants and products are separated, isolated, and pure. Furthermore, the reactants and products are all at the same temperature ...
... U = U products U reactants and H = H products H reactants. One thing is sometimes not made very clear. "Reactants" and "products" in these equations means that the reactants and products are separated, isolated, and pure. Furthermore, the reactants and products are all at the same temperature ...
Paper - Edexcel
... A The student used a higher temperature than in the other experiments. B The student used less copper(II) carbonate than in the other experiments. C The student heated the crucible without a lid on. D The student used a spirit burner instead of a Bunsen burner. (d) In another experiment, the student ...
... A The student used a higher temperature than in the other experiments. B The student used less copper(II) carbonate than in the other experiments. C The student heated the crucible without a lid on. D The student used a spirit burner instead of a Bunsen burner. (d) In another experiment, the student ...
Chemistry 130 Physical + Chemical Change
... Consider the reactants (stuff you started with) in procedure 1. Magnesium metal and oxygen gas (from the air). Please write the electron gain and loss process equations for this reaction [these two equations will be of the form immediately above for the copper(II) ion and zinc metal reaction]. Reduc ...
... Consider the reactants (stuff you started with) in procedure 1. Magnesium metal and oxygen gas (from the air). Please write the electron gain and loss process equations for this reaction [these two equations will be of the form immediately above for the copper(II) ion and zinc metal reaction]. Reduc ...
9.2 Group II
... All Group II elements react with acids to form the corresponding salts and hydrogen gas. Beryllium does not react with acids at room temperature. It reacts slowly at higher temperatures. Magnesium, calcium, strontium and barium react vigorously with acids to form the corresponding ion and hydr ...
... All Group II elements react with acids to form the corresponding salts and hydrogen gas. Beryllium does not react with acids at room temperature. It reacts slowly at higher temperatures. Magnesium, calcium, strontium and barium react vigorously with acids to form the corresponding ion and hydr ...
FONTARGEN AP 22LL
... FONTARGEN AP 22LL is a flux-free, slow-drying copper-tin hard solder paste with a medium viscosity and medium metal content. AP 22LL can be used in hydrogen-nitrogen mixtures or exothermic atmospheres. Thanks to its low melting temperature range, the paste is suited to brazing of work pieces made of ...
... FONTARGEN AP 22LL is a flux-free, slow-drying copper-tin hard solder paste with a medium viscosity and medium metal content. AP 22LL can be used in hydrogen-nitrogen mixtures or exothermic atmospheres. Thanks to its low melting temperature range, the paste is suited to brazing of work pieces made of ...
TEST#2
... (a). Which of the three gases, neon, oxygen and chlorine, which one has the lowest boiling point and which has the highest boiling point? (b). Which of the substances listed are liquids at 62˚C? (c). If a mixture of gold and copper where heated to a temperature of 2,600˚C, which of the two metals wo ...
... (a). Which of the three gases, neon, oxygen and chlorine, which one has the lowest boiling point and which has the highest boiling point? (b). Which of the substances listed are liquids at 62˚C? (c). If a mixture of gold and copper where heated to a temperature of 2,600˚C, which of the two metals wo ...
Word Pro
... (b) What is the empirical formula for the compound? 12. Chromium metal can be produced in an electric furnace by the reaction: 2 Cr2O3(s) + 3 Si(s) + 3 CaO(s) → 4 Cr(s) + 3 CaSiO3(s) (a) How many mole of Cr2O3(s) are needed to make 5 mole of Cr(s). (b) How many mole of silicon, Si(s), are used in ma ...
... (b) What is the empirical formula for the compound? 12. Chromium metal can be produced in an electric furnace by the reaction: 2 Cr2O3(s) + 3 Si(s) + 3 CaO(s) → 4 Cr(s) + 3 CaSiO3(s) (a) How many mole of Cr2O3(s) are needed to make 5 mole of Cr(s). (b) How many mole of silicon, Si(s), are used in ma ...
From discrete or polymeric heterometallic complexes to the mixed
... use of mixed-metal oxides as sensors, actuators, and smart materials has also been explored. It is known that the effect of crystallinity, particle size, structure and morphology of these materials could highly affect their properties. These can be tuned in part by changing the synthesis methods. Th ...
... use of mixed-metal oxides as sensors, actuators, and smart materials has also been explored. It is known that the effect of crystallinity, particle size, structure and morphology of these materials could highly affect their properties. These can be tuned in part by changing the synthesis methods. Th ...
chemistry 102 fall 2001 part 1
... (3) Do NOT write on the envelope. (4) Bubble in OPTION A on the scanning sheet IF you want your grade posted. (5) When finished, put the free response answers in the envelope with the scanning sheet. You can keep the multiple choice part - the answers will be given to you as you leave. (6) There are ...
... (3) Do NOT write on the envelope. (4) Bubble in OPTION A on the scanning sheet IF you want your grade posted. (5) When finished, put the free response answers in the envelope with the scanning sheet. You can keep the multiple choice part - the answers will be given to you as you leave. (6) There are ...
Abstract
... The use of first row transition metals in catalysis as a substitute for scarce and expensive second and third row transition metals is still a challenge One strategy to promote the use of first row transition metals is the use of bimetallic complexes as the cooperativity between the metal centers mi ...
... The use of first row transition metals in catalysis as a substitute for scarce and expensive second and third row transition metals is still a challenge One strategy to promote the use of first row transition metals is the use of bimetallic complexes as the cooperativity between the metal centers mi ...
Types of Chemical Reactions
... • If the combustion is complete, the products will be CO2 and H2O. • If the combustion is incomplete, , the products will be CO (or possibly just C) and H2O. ...
... • If the combustion is complete, the products will be CO2 and H2O. • If the combustion is incomplete, , the products will be CO (or possibly just C) and H2O. ...
Unit 10
... Determine the types of reactants involved and the products formed in the reaction. Write down the correct formulae of reactants on the left hand side of the arrow. Write down the correct formulae of products on the right hand side of the arrow. Balance the equation with simple whole numbers such tha ...
... Determine the types of reactants involved and the products formed in the reaction. Write down the correct formulae of reactants on the left hand side of the arrow. Write down the correct formulae of products on the right hand side of the arrow. Balance the equation with simple whole numbers such tha ...
Unit 7 Review
... Which of the following metals could be added to aqueous iron (II) oxide to produce solid iron metal? ...
... Which of the following metals could be added to aqueous iron (II) oxide to produce solid iron metal? ...
LIMITING REACTANT LAB
... d. If you want a refresher, there’s a pencast on this material. Questions will be similar to the D2 Quiz and to the chemical names on the problem sets. 2. Reaction balancing a. Be able to balance chemical reactions. 3. Stoichiometry (Chp. 12) a. Be able to compute the amount (in either grams or mole ...
... d. If you want a refresher, there’s a pencast on this material. Questions will be similar to the D2 Quiz and to the chemical names on the problem sets. 2. Reaction balancing a. Be able to balance chemical reactions. 3. Stoichiometry (Chp. 12) a. Be able to compute the amount (in either grams or mole ...
Two metals of equal mass with different heat capacities are
... 1) The metal with the higher heat capacity. 2) The metal with the lower heat capacity. 3) Both undergo the same change in temperature. 4) You need to know the initial temp of the metals. ...
... 1) The metal with the higher heat capacity. 2) The metal with the lower heat capacity. 3) Both undergo the same change in temperature. 4) You need to know the initial temp of the metals. ...
Final exam review - Iowa State University
... 8. A sample of CO2 gas has a pressure of 56.5 mm Hg in a 125 mL flask. The sample is transferred to a new flask, where it has a pressure of 62.3 mm Hg at the same temperature. What is the volume of the new flask? ...
... 8. A sample of CO2 gas has a pressure of 56.5 mm Hg in a 125 mL flask. The sample is transferred to a new flask, where it has a pressure of 62.3 mm Hg at the same temperature. What is the volume of the new flask? ...
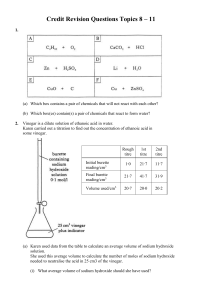
Credit Revision Questions Topics 8 – 11 1. (a) Which box contains a
... It reacts with cold water. ...
... It reacts with cold water. ...
Chapter 5 - Angelo State University
... 13. Mithril is a tough, lightweight metal mined by the dwarves in the mines of Moria. A 25.00 g sample of mithril is heated in a test tube to 100.00°C in boiling water and carefully added to a coffee-cup calorimeter containing 50.00 g of water. The water temperature increased from 25.50°C to 30.50°C ...
... 13. Mithril is a tough, lightweight metal mined by the dwarves in the mines of Moria. A 25.00 g sample of mithril is heated in a test tube to 100.00°C in boiling water and carefully added to a coffee-cup calorimeter containing 50.00 g of water. The water temperature increased from 25.50°C to 30.50°C ...
Thermochemistry Questions
... carbon. The balanced reaction looks like this: • 2Mg (s) + CO2(s) 2 MgO (s) + C(S) • Using the enthalpy data provided on the sheet, calculate the enthalpy change for this reaction (H rxn ) in kJ. ...
... carbon. The balanced reaction looks like this: • 2Mg (s) + CO2(s) 2 MgO (s) + C(S) • Using the enthalpy data provided on the sheet, calculate the enthalpy change for this reaction (H rxn ) in kJ. ...
inorganic-chemistry-gp-i-alkali-metals
... The stability of peroxides and super oxides increases moving down the group. Li here also shows an anomalous behaviour, when react with air it is the only metal to react with N2 present. Li + Air Li2O + Li3N the here also driving force is high lattice energy of product. Li3N + H2O LiOH + NH3 ...
... The stability of peroxides and super oxides increases moving down the group. Li here also shows an anomalous behaviour, when react with air it is the only metal to react with N2 present. Li + Air Li2O + Li3N the here also driving force is high lattice energy of product. Li3N + H2O LiOH + NH3 ...
Unit 2 Section B Supplement b
... Section B Supplement In which layer of the earth are the following resources found: a. Water? ...
... Section B Supplement In which layer of the earth are the following resources found: a. Water? ...
Thermite

Thermite is a pyrotechnic composition of metal powder fuel and metal oxide. When ignited by heat, thermite undergoes an exothermic reduction-oxidation (redox) reaction. Most varieties are not explosive but can create brief bursts of high temperature in a small area. Its form of action is similar to that of other fuel-oxidizer mixtures, such as black powder.Thermites have diverse compositions. Fuels include aluminium, magnesium, titanium, zinc, silicon, and boron. Aluminium is common because of its high boiling point and low cost. Oxidizers include bismuth(III) oxide, boron(III) oxide, silicon(IV) oxide, chromium(III) oxide, manganese(IV) oxide, iron(III) oxide, iron(II,III) oxide, copper(II) oxide, and lead(II,IV) oxide.The reaction is used for thermite welding, often used to join rail tracks. Thermites have also been used in metal refining, demolition of munitions, and in incendiary weapons. Some thermite-like mixtures are used as pyrotechnic initiators in fireworks.