Balancing Chemical Equations – A Primer
... Say what? These are two elements that SHARE electrons. This is called a COVALENT BOND. For the moment, these are elements located on the right side of the Periodic Table. Thus, two negatively charged elements bonded. How do two negatives stay together...that breaks the rule that like-charges repel? ...
... Say what? These are two elements that SHARE electrons. This is called a COVALENT BOND. For the moment, these are elements located on the right side of the Periodic Table. Thus, two negatively charged elements bonded. How do two negatives stay together...that breaks the rule that like-charges repel? ...
Chapter 4 Nomenclature and Chemical Equations
... It is a convention to write the reactants on the left and the products on the right. The letters enclosed in the parenthesis tell us the states of the substances: s denotes a solid, l denotes a liquid, g denotes a gas and aq denotes an aqueous solution, i.e. a homogeneous mixture in water. Theref ...
... It is a convention to write the reactants on the left and the products on the right. The letters enclosed in the parenthesis tell us the states of the substances: s denotes a solid, l denotes a liquid, g denotes a gas and aq denotes an aqueous solution, i.e. a homogeneous mixture in water. Theref ...
Strumenti tutor LIM
... We can realize that a chemical reaction is taking place when...........( there is a change in colour, a gas or a solid is formed in a solution, heat is produced or the system is cooled down,.) A chemical equation is balanced when........................(the number of atoms of each species is the sam ...
... We can realize that a chemical reaction is taking place when...........( there is a change in colour, a gas or a solid is formed in a solution, heat is produced or the system is cooled down,.) A chemical equation is balanced when........................(the number of atoms of each species is the sam ...
Spears® Thermoplastic Piping System Materials - r-c
... application and system design. To avoid problems, the following key points must be considered when selecting materials for an application and in designing a system for their use. 1. Fluid incompatibility of certain chemicals, especially petroleum distillates and derivatives, can cause environmental ...
... application and system design. To avoid problems, the following key points must be considered when selecting materials for an application and in designing a system for their use. 1. Fluid incompatibility of certain chemicals, especially petroleum distillates and derivatives, can cause environmental ...
The Shale Gas Revolution: A Methane-to
... technolog is needed to utilize tili e all components of shale gas for organic chemical production? EES — 15 ...
... technolog is needed to utilize tili e all components of shale gas for organic chemical production? EES — 15 ...
Unit 8 Test Review
... Apply the Law of Conservation of Mass to get the same number of atoms of every element on each side of the equation. Tip: Start by balancing an element that appears in only one reactant and product. Once one element is balanced, proceed to balance another, and another, until all elements are balance ...
... Apply the Law of Conservation of Mass to get the same number of atoms of every element on each side of the equation. Tip: Start by balancing an element that appears in only one reactant and product. Once one element is balanced, proceed to balance another, and another, until all elements are balance ...
7.1 Describing Reactions
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
Slide 1
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
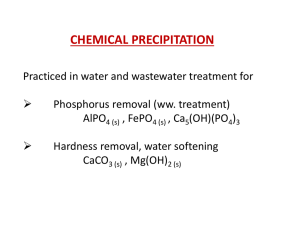
Phosphorus Removal from Wastewater by Chemical Precipitation
... • A reasonably good correlation can be obtained by plotting the log of the fraction of soluble phosphorus remaining as a function of the weight ratio of metal to soluble phosphorus. • The required ratio of metal ion to influent soluble phosphorus for a specified phosphorus removal can be obtained fr ...
... • A reasonably good correlation can be obtained by plotting the log of the fraction of soluble phosphorus remaining as a function of the weight ratio of metal to soluble phosphorus. • The required ratio of metal ion to influent soluble phosphorus for a specified phosphorus removal can be obtained fr ...
7.1 Describing Reactions
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
7.1 Describing Reactions
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
... Chemical Equations Conservation of Mass During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle is the law of conservation of mass. When charcoal burns, the mass of the carbon dioxide produced is equal to the mass of the charcoal and oxygen th ...
Chemistry General v. 2016
... General Chemistry This chemistry course is designed to provide students with an opportunity to develop and evaluate basic ideas and theories related to chemistry. Students are required to apply skills and knowledge to chemistry principles in order to explain various concepts, solve problems, and com ...
... General Chemistry This chemistry course is designed to provide students with an opportunity to develop and evaluate basic ideas and theories related to chemistry. Students are required to apply skills and knowledge to chemistry principles in order to explain various concepts, solve problems, and com ...
Characterization of Multi-constituent Substances for REACH
... points, phase transitions and decompositions. Many chemical analytical methods have been developed for measuring specific constituents and can distinguish between, for example, nitrate nitrogen and ammoniacal nitrogen. ADDITIONAL INFORMATION In addition to the actual analytical testing, it is import ...
... points, phase transitions and decompositions. Many chemical analytical methods have been developed for measuring specific constituents and can distinguish between, for example, nitrate nitrogen and ammoniacal nitrogen. ADDITIONAL INFORMATION In addition to the actual analytical testing, it is import ...
Chemistry in engineering curriculum Prisedsky V.V. (DonNTU
... optically active centres, that define the properties of functional crystalline materials for use in electronics, are controlled by the very same laws of chemical thermodynamics and kinetics. d) Chemical thermodynamics and kinetics are basic components of the theory of technological processes dealing ...
... optically active centres, that define the properties of functional crystalline materials for use in electronics, are controlled by the very same laws of chemical thermodynamics and kinetics. d) Chemical thermodynamics and kinetics are basic components of the theory of technological processes dealing ...
CH100: Fundamentals for Chemistry
... Mixtures can be separated by physical means (and also by chemical methods, as well) There are 2 general methods of separation Physical separation Chemical separation ...
... Mixtures can be separated by physical means (and also by chemical methods, as well) There are 2 general methods of separation Physical separation Chemical separation ...
Chemical Reaction Th..
... breaking / reformation of chemical bonds out-giving or in-taking heat ...
... breaking / reformation of chemical bonds out-giving or in-taking heat ...
5.12 Lab Design and Safety
... o Chemical, biological, and radiological wastes are required to be stored in the lab in which they are generated, not in centralized accumulation areas. They are periodically collected and sent to a disposal/ recycling center. Although chemical hygiene plans prohibit dumping chemicals into the drain ...
... o Chemical, biological, and radiological wastes are required to be stored in the lab in which they are generated, not in centralized accumulation areas. They are periodically collected and sent to a disposal/ recycling center. Although chemical hygiene plans prohibit dumping chemicals into the drain ...
blah
... The most component in feedstock is lignin. The percentage is more than 50%. The discussion is focused on lignin. After activation, almost no sugar carbon left in the residue. Point 8:Figure 5. It would be important to have, maybe noted in the same figures, also the result from ...
... The most component in feedstock is lignin. The percentage is more than 50%. The discussion is focused on lignin. After activation, almost no sugar carbon left in the residue. Point 8:Figure 5. It would be important to have, maybe noted in the same figures, also the result from ...
2011-2012 ACAD REVIEW SHEET Chapter 2
... What is the relationship between the kinetic energy of molecules and their physical state? (ANS: Gases have the greatest amount of kinetic energy; solids have the least.) ...
... What is the relationship between the kinetic energy of molecules and their physical state? (ANS: Gases have the greatest amount of kinetic energy; solids have the least.) ...
Section 1 Describing Chemical Reactions Chapter 8
... Solid aluminum carbide, Al4C3, reacts with water to produce methane gas and solid aluminum hydroxide. Write a balanced chemical equation for this reaction. ...
... Solid aluminum carbide, Al4C3, reacts with water to produce methane gas and solid aluminum hydroxide. Write a balanced chemical equation for this reaction. ...
What Can I Do With a Major In Chemistry
... Chemistry is the study of properties’ composition, changes and use of matter. Chemistry is divided into five main areas: analytical chemistry, biochemistry, inorganic chemistry, organic chemistry and physical chemistry. Analytical chemistry is the study of the physical and chemical properties of com ...
... Chemistry is the study of properties’ composition, changes and use of matter. Chemistry is divided into five main areas: analytical chemistry, biochemistry, inorganic chemistry, organic chemistry and physical chemistry. Analytical chemistry is the study of the physical and chemical properties of com ...
Chemistry: Matter and Change
... two, or three-letter symbol. • The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups. ...
... two, or three-letter symbol. • The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups. ...
the properties and structure of matter
... • Phase change – transition of a substance from one state to another – Depend on temperature and pressure – Affects: » Affects particle arrangement » Energy of particles » Distance between particles ...
... • Phase change – transition of a substance from one state to another – Depend on temperature and pressure – Affects: » Affects particle arrangement » Energy of particles » Distance between particles ...
Chemistry Lesson Plans #07 - Chemical Reactions
... chemical reaction – they are merely rearranged. – Remember the Law of Conservation of Mass? o In describing a chemical reaction you use statements such as Iron reacts with oxygen to produce iron(III) oxide which is called rust Or it could be written iron + oxygen iron(III) oxide • Note that the reac ...
... chemical reaction – they are merely rearranged. – Remember the Law of Conservation of Mass? o In describing a chemical reaction you use statements such as Iron reacts with oxygen to produce iron(III) oxide which is called rust Or it could be written iron + oxygen iron(III) oxide • Note that the reac ...
Chemical plant
A chemical plant is an industrial process plant that manufactures (or otherwise processes) chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transformation and or separation of materials. Chemical plants use specialized equipment, units, and technology in the manufacturing process. Other kinds of plants, such as polymer, pharmaceutical, food, and some beverage production facilities, power plants, oil refineries or other refineries, natural gas processing and biochemical plants, water and wastewater treatment, and pollution control equipment use many technologies that have similarities to chemical plant technology such as fluid systems and chemical reactor systems. Some would consider an oil refinery or a pharmaceutical or polymer manufacturer to be effectively a chemical plant.Petrochemical plants (plants using chemicals from petroleum as a raw material or feedstock ) are usually located adjacent to an oil refinery to minimize transportation costs for the feedstocks produced by the refinery. Speciality chemical and fine chemical plants are usually much smaller and not as sensitive to location. Tools have been developed for converting a base project cost from one geographic location to another.