Biology Management Options Kinnikinnick: Leaf gall
... Leaf gall of kinnikinnick is caused by the same fungus which causes leaf and flower gall of azalea. Initially, infected plant parts show a thickening and then gradually become fleshy in appearance. Infected leaves and flowers thicken into greenish to pinkish galls. As the galls mature, they become c ...
... Leaf gall of kinnikinnick is caused by the same fungus which causes leaf and flower gall of azalea. Initially, infected plant parts show a thickening and then gradually become fleshy in appearance. Infected leaves and flowers thicken into greenish to pinkish galls. As the galls mature, they become c ...
The Periodic table and subatomic particles
... Have a pH greater than 7 Taste bitter and feel slippery (*NOTE: do not taste or touch in the lab) Have a pH less than 7 React with active metals to produce H2(g) ...
... Have a pH greater than 7 Taste bitter and feel slippery (*NOTE: do not taste or touch in the lab) Have a pH less than 7 React with active metals to produce H2(g) ...
Chapter 2 - Cloudfront.net
... Weigh mass before and after change. Mass before and after is always the same. ...
... Weigh mass before and after change. Mass before and after is always the same. ...
Protecting Buildings from Chemical and Biological Warfare Agent
... features that no human immune system has ever seen before. This ability to engineer a new cell surface also means that current antibody-based sensors can also be easily evaded, and even individuals who have been immunized against known forms of a pathogen could be susceptible to an attack. Other exa ...
... features that no human immune system has ever seen before. This ability to engineer a new cell surface also means that current antibody-based sensors can also be easily evaded, and even individuals who have been immunized against known forms of a pathogen could be susceptible to an attack. Other exa ...
Chapter 13 Notes
... color, melting point, electrical conductivity and specific heat are examples of physical properties. Chemical properties describe how matter can undergo changes in composition either by itself or as it interacts with other matter. A chemical property of wood or paper is that it undergoes the chemica ...
... color, melting point, electrical conductivity and specific heat are examples of physical properties. Chemical properties describe how matter can undergo changes in composition either by itself or as it interacts with other matter. A chemical property of wood or paper is that it undergoes the chemica ...
Name
... Essential Standard 9f: Apply simple mathematical relationships to determine one quantity given the other two (including speed= distance x time, density = mass/volume, force = pressure x area, volume = area x height). ...
... Essential Standard 9f: Apply simple mathematical relationships to determine one quantity given the other two (including speed= distance x time, density = mass/volume, force = pressure x area, volume = area x height). ...
MSDS - Firestone Building Products
... Steps to Be Taken in Case Material is Released or Spilled: ...
... Steps to Be Taken in Case Material is Released or Spilled: ...
HYDRA-HUME
... CHILDREN. DO NOT STORE WITH FOOD, FEED, OR OTHER MATERIAL TO BE USED OR CONSUMED BY HUMANS OR ANIMALS. DO NOT CONTAMINATE WATER SUPPLIES, LAKES, STREAMS, ...
... CHILDREN. DO NOT STORE WITH FOOD, FEED, OR OTHER MATERIAL TO BE USED OR CONSUMED BY HUMANS OR ANIMALS. DO NOT CONTAMINATE WATER SUPPLIES, LAKES, STREAMS, ...
[Mg] +2[ S ]-2
... 6. Burning toast in the toaster chemical reaction 7. Chopping up firewood not a chemical reaction 8. Mixing red and blue paint together in order to get purple not a chemical reaction 9. Blowing bubbles through a straw in a glass of chocolate milk not a chemical reaction 10. Crystals forming when mak ...
... 6. Burning toast in the toaster chemical reaction 7. Chopping up firewood not a chemical reaction 8. Mixing red and blue paint together in order to get purple not a chemical reaction 9. Blowing bubbles through a straw in a glass of chocolate milk not a chemical reaction 10. Crystals forming when mak ...
Chapter 10_Handouts_6
... in many compounds and remain together during chemical reactions. The sulfate group SO4 is an example of an atom group. A precipitate is an insoluble solid that results from a chemical reaction in solution. When two or more atom groups of the same kind are present in the formula of a compound, parent ...
... in many compounds and remain together during chemical reactions. The sulfate group SO4 is an example of an atom group. A precipitate is an insoluble solid that results from a chemical reaction in solution. When two or more atom groups of the same kind are present in the formula of a compound, parent ...
Chapter 10 Handouts - Bakersfield College
... 10-7. The Periodic Table The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the elemen ...
... 10-7. The Periodic Table The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the elemen ...
Chapter 10 Handouts_1
... 10-7. The Periodic Table The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the eleme ...
... 10-7. The Periodic Table The Russian chemist Dmitri Mendeleev formulated the periodic law about 1869 which states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular intervals. The periodic table is a listing of the eleme ...
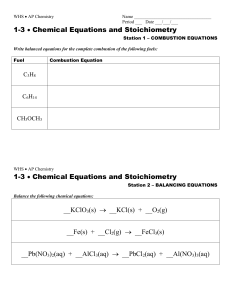
Types of Chemical Reactions Name_________________________
... Note: Access to some of the websites may not be possible dependent upon your computer system and or the network connection. You are still required to work through each main type of reaction providing balanced chemical equations based on the word descriptions. I. How can I tell if a chemical reaction ...
... Note: Access to some of the websites may not be possible dependent upon your computer system and or the network connection. You are still required to work through each main type of reaction providing balanced chemical equations based on the word descriptions. I. How can I tell if a chemical reaction ...
Intro to Chem
... ◦ During any chemical rxn, the mass of the products is always equal to the mass of the reactants. ◦ Law of Conservation of Mass – states that in any physical change or chemical rxn, mass is conserved. ...
... ◦ During any chemical rxn, the mass of the products is always equal to the mass of the reactants. ◦ Law of Conservation of Mass – states that in any physical change or chemical rxn, mass is conserved. ...
one
... • During a chemical reaction, chemical bonds in the reactants are broken and chemical bonds in the products are formed. – Breaking bonds requires energy. – Forming bonds releases energy. ...
... • During a chemical reaction, chemical bonds in the reactants are broken and chemical bonds in the products are formed. – Breaking bonds requires energy. – Forming bonds releases energy. ...
Ch. 3 9-Station Review
... Station 8 – PERCENT YIELD Solve the following problem: Hydrogen gas was generated according to the equation: Zn(s) + 2HCl(aq) H2(g) + ZnCl2(aq) When 25.00 grams of Zn metal reacted with excess HCl 7.50 L H2(g) was collected at STP. The theoretical yield of H2(g) for this reaction is: (show work) ...
... Station 8 – PERCENT YIELD Solve the following problem: Hydrogen gas was generated according to the equation: Zn(s) + 2HCl(aq) H2(g) + ZnCl2(aq) When 25.00 grams of Zn metal reacted with excess HCl 7.50 L H2(g) was collected at STP. The theoretical yield of H2(g) for this reaction is: (show work) ...
7.5.9 Compare physical properties of matter to the chemical property
... The temperature at which a solid can change to a liquid The temperature at which a pure substance melts is unchanging under constant conditions The melting point of a pure substance can be used as a physical property for identification. For example ice melts to form liquid water at 0 degrees Cels ...
... The temperature at which a solid can change to a liquid The temperature at which a pure substance melts is unchanging under constant conditions The melting point of a pure substance can be used as a physical property for identification. For example ice melts to form liquid water at 0 degrees Cels ...
3 CO 2(g)
... Properties of original substance disappear as new substances with different properties are formed Change in chemical composition Cannot return to original form Can be detected through – energy changes (temperature), change in color, emission of gas, solid formed ...
... Properties of original substance disappear as new substances with different properties are formed Change in chemical composition Cannot return to original form Can be detected through – energy changes (temperature), change in color, emission of gas, solid formed ...
Honors Midterm - Stamford High School
... 3. Make an element inventory. How are you going to know if the equation is balanced if you don't actually make a list of how many of each atom you have? You won't. You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole prob ...
... 3. Make an element inventory. How are you going to know if the equation is balanced if you don't actually make a list of how many of each atom you have? You won't. You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole prob ...
Drug Testing - Uplift Grand
... (there are a few VERY similar chemicals that cannot be differentiated via GC-MS, such as. stereoisomers) Watch me! ...
... (there are a few VERY similar chemicals that cannot be differentiated via GC-MS, such as. stereoisomers) Watch me! ...
Chapter 1 - Manual Science Chemistry/Physics
... Matter – anything that has mass and takes up space. Atom – smallest unit of an element that maintains the chemical identity of that element. Molecule – smallest stable unit of a substance; can be composed of one or more kinds of atoms. Element – a pure substance that cannot be broken down in ...
... Matter – anything that has mass and takes up space. Atom – smallest unit of an element that maintains the chemical identity of that element. Molecule – smallest stable unit of a substance; can be composed of one or more kinds of atoms. Element – a pure substance that cannot be broken down in ...
Chapter One Powerpoint - Geneva Area City Schools
... • An atom is the smallest unit of an element that maintains the chemical identity of that element. • Fundamental building block of matter • An element is a pure substance that cannot be broken down into simpler, more stable substances and is made of one type of atom. • A compound is a substance that ...
... • An atom is the smallest unit of an element that maintains the chemical identity of that element. • Fundamental building block of matter • An element is a pure substance that cannot be broken down into simpler, more stable substances and is made of one type of atom. • A compound is a substance that ...
Chemical plant
A chemical plant is an industrial process plant that manufactures (or otherwise processes) chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transformation and or separation of materials. Chemical plants use specialized equipment, units, and technology in the manufacturing process. Other kinds of plants, such as polymer, pharmaceutical, food, and some beverage production facilities, power plants, oil refineries or other refineries, natural gas processing and biochemical plants, water and wastewater treatment, and pollution control equipment use many technologies that have similarities to chemical plant technology such as fluid systems and chemical reactor systems. Some would consider an oil refinery or a pharmaceutical or polymer manufacturer to be effectively a chemical plant.Petrochemical plants (plants using chemicals from petroleum as a raw material or feedstock ) are usually located adjacent to an oil refinery to minimize transportation costs for the feedstocks produced by the refinery. Speciality chemical and fine chemical plants are usually much smaller and not as sensitive to location. Tools have been developed for converting a base project cost from one geographic location to another.







![[Mg] +2[ S ]-2](http://s1.studyres.com/store/data/014450548_1-468f3af464a09baae245d79fadf97d41-300x300.png)