Disclosures
... • Only two randomized, placebocontrolled trials with BMI as prespecified endpoint – Study in adolescents 13-18 years old reported 3% BMI reduction – Study in children ages 6-12 years old reported 3.2% BMI reduction ...
... • Only two randomized, placebocontrolled trials with BMI as prespecified endpoint – Study in adolescents 13-18 years old reported 3% BMI reduction – Study in children ages 6-12 years old reported 3.2% BMI reduction ...
Management of diabetes
... • Diabetic keto-acidosis • Hyperosmolar states • Hypoglycemia with neuroglycopenia • Recurrent or persistent poor glycemic control • Severe chronic complications of diabetes • Initiation of intensive insulin regimens ...
... • Diabetic keto-acidosis • Hyperosmolar states • Hypoglycemia with neuroglycopenia • Recurrent or persistent poor glycemic control • Severe chronic complications of diabetes • Initiation of intensive insulin regimens ...
Classification of diabetes
... Type 2 Diabetes Mellitus. Although most present in middle and old age, an increasing number present at younger ages. Many are obese. Peripheral insulin resistance combined with relative insulin deficiency accounts for the pathogenesis. Ketonuria is absent in the non-fasting state. Such patients are ...
... Type 2 Diabetes Mellitus. Although most present in middle and old age, an increasing number present at younger ages. Many are obese. Peripheral insulin resistance combined with relative insulin deficiency accounts for the pathogenesis. Ketonuria is absent in the non-fasting state. Such patients are ...
Low-cost vaccine could reverse juvenile diabetes
... A low-cost tuberculosis vaccine that's been in use for decades could get new life as a treatment for juvenile diabetes -- with preliminary research showing it could reverse the disease. A group of Boston scientists have been testing bacillus Calmette-Guerin (BSG), a vaccine used for more than eight ...
... A low-cost tuberculosis vaccine that's been in use for decades could get new life as a treatment for juvenile diabetes -- with preliminary research showing it could reverse the disease. A group of Boston scientists have been testing bacillus Calmette-Guerin (BSG), a vaccine used for more than eight ...
ABSTRACT Title: Possibilities, how patients NIDDM can use
... four characteristic points for motion training: a form of exercise, intensity of exercise, type of exercise and duration of exercise, known by the acronym F.I.T.T. These points are evaluated individually due to the patient with the disease diabetes mellitus II. ...
... four characteristic points for motion training: a form of exercise, intensity of exercise, type of exercise and duration of exercise, known by the acronym F.I.T.T. These points are evaluated individually due to the patient with the disease diabetes mellitus II. ...
ICTR CONNECTIONS AUGUST, 2013
... Rally for Medical Research Hill Day September 18, 2013 Washington, DC A Comparative Effectiveness Study Jill Crandall, MD and Diane McKee, MD are spearheading this study at Einstein-Montefiore, as part of an NIH project involving 37 medical centers nationally, to determine the best combination drug ...
... Rally for Medical Research Hill Day September 18, 2013 Washington, DC A Comparative Effectiveness Study Jill Crandall, MD and Diane McKee, MD are spearheading this study at Einstein-Montefiore, as part of an NIH project involving 37 medical centers nationally, to determine the best combination drug ...
diabetics
... • Glibenclamide should be avoided for newly diagnosed cases of type 2 diabetes in older adults (>70 years ) because of the marked risk of hypoglycaemia. Less risk :Glipizide , Glimepride. • A DPP-4 inhibitor as an add-on to metformin when use of a sulphonylurea may pose an unacceptable hypoglycaemi ...
... • Glibenclamide should be avoided for newly diagnosed cases of type 2 diabetes in older adults (>70 years ) because of the marked risk of hypoglycaemia. Less risk :Glipizide , Glimepride. • A DPP-4 inhibitor as an add-on to metformin when use of a sulphonylurea may pose an unacceptable hypoglycaemi ...
Periodic Height, weight, BMI, [A] assessment
... Comprehensive diabetes self-management education and support (DSME and DSMS) from a collaborative team or diabetic counseling and risk educator if available factor modification Education should be individualized, based on the National Standards for DSME1[B] and include: Importance of regular physica ...
... Comprehensive diabetes self-management education and support (DSME and DSMS) from a collaborative team or diabetic counseling and risk educator if available factor modification Education should be individualized, based on the National Standards for DSME1[B] and include: Importance of regular physica ...
Slide ()
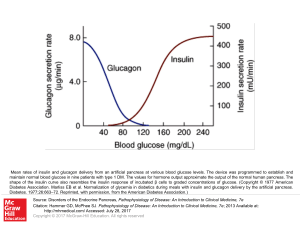
... Mean rates of insulin and glucagon delivery from an artificial pancreas at various blood glucose levels. The device was programmed to establish and maintain normal blood glucose in nine patients with type 1 DM. The values for hormone output approximate the output of the normal human pancreas. The sh ...
... Mean rates of insulin and glucagon delivery from an artificial pancreas at various blood glucose levels. The device was programmed to establish and maintain normal blood glucose in nine patients with type 1 DM. The values for hormone output approximate the output of the normal human pancreas. The sh ...
127 Killers of Century DM-HTN&CAD
... GTT 2-hour blood sugar level during a 75-g oral glucose tolerance test. ...
... GTT 2-hour blood sugar level during a 75-g oral glucose tolerance test. ...
Gemigliptin
Gemigliptin (rINN), previously identified as LC15-0444, is an oral anti-hyperglycemic agent (anti-diabetic drug) of the new dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. It is well known that glucose lowering effects of DPP-4 inhibitors are mainly mediated by GLP-1 and gastric inhibitory polypeptide (GIP) incretin hormones which are inactivated by DPP-4.Gemigliptin was initially developed solely by LG Life Sciences. In 2010, Double-Crane Pharmaceutical Co. (DCPC) joined with LGLS to co-develop the final compound and collaborate on the marketing of the drug in China. LGLS also announced on Nov., 2010 that NOBEL Ilac has been granted rights to develop and commercialize gemigliptin in Turkey.A New Drug Application (NDA) for gemigliptin in the treatment of type 2 diabetes was submitted to the Korea Food & Drug Administration (KFDA) in July 2011. Then on June 27, 2012, the KFDA has approved the manufacture and distribution of LG Life Sciences’ diabetes treatment, Zemiglo, the main substance of which is gemigliptin. Clinical trials for evaluating the safety and efficacy of gemigliptin in combination with metformin have been completed.








![Periodic Height, weight, BMI, [A] assessment](http://s1.studyres.com/store/data/008718203_1-0c83d238fd18dca05a07bfd7a4536266-300x300.png)