Homo-coupling of terminal alkynes on a noble metal surface
... implies a reaction barrier of 1.8 eV, being rather high compared with the overall energy barrier of 0.38 eV identified for the Ullmann reaction of C6H5 units on Cu(111) that also proceeds at mild temperatures31. An alternative, putative mechanism via an adatom-related dehydrogenation (Fig. 4b) simila ...
... implies a reaction barrier of 1.8 eV, being rather high compared with the overall energy barrier of 0.38 eV identified for the Ullmann reaction of C6H5 units on Cu(111) that also proceeds at mild temperatures31. An alternative, putative mechanism via an adatom-related dehydrogenation (Fig. 4b) simila ...
Document
... 21. If 2.891 g MgCl2 is dissolved in enough water to make 500.0 mL of solution, what is the molarity of the magnesium chloride solution? ...
... 21. If 2.891 g MgCl2 is dissolved in enough water to make 500.0 mL of solution, what is the molarity of the magnesium chloride solution? ...
Second Year - WordPress.com
... Dobreiner’s work led to the law of triads which states that ______ a) Atomic weight of any one element was found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Ato ...
... Dobreiner’s work led to the law of triads which states that ______ a) Atomic weight of any one element was found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Ato ...
Computational Study of protonation of ozone
... atm) are derived from the zero-point energy, as well as the corresponding-thermal corrections to the electron energy. Results and discussions Early studies [8] have shown that ozone can have singlet and triplet electronic structure. We have made calculations of the geometric characteristics of the p ...
... atm) are derived from the zero-point energy, as well as the corresponding-thermal corrections to the electron energy. Results and discussions Early studies [8] have shown that ozone can have singlet and triplet electronic structure. We have made calculations of the geometric characteristics of the p ...
- Kendriya Vidyalaya No. 2 Raipur
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and otherproperties. ...
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and otherproperties. ...
CHAPTER TWO SOLID STATE REACTIONS 2.0 Introduction The
... phenylacetophenone the solvent-free, mechanically induced, transformation results in the formation of the thermodynamically favorable C-phosphorylated product, which in solution is only obtained together with side products [55]. The mechanical preparation of phosphorus ylides has also been reported ...
... phenylacetophenone the solvent-free, mechanically induced, transformation results in the formation of the thermodynamically favorable C-phosphorylated product, which in solution is only obtained together with side products [55]. The mechanical preparation of phosphorus ylides has also been reported ...
class XI CHEMISTRY - Kendriya Vidyalaya No.1 Ichhanath Surat
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and otherproperties. ...
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and otherproperties. ...
class XI CHEMISTRY - Kendriya Vidyalaya No.1 Harni Road
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and otherproperties. ...
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and otherproperties. ...
Document
... Calorimetry is an accurate technique for determining enthalpy changes, but how do chemists deal with chemical systems that cannot be analyzed using this technique? For example, the rusting of iron (Figure 1) is extremely slow and, therefore, the resulting temperature change would be too small to be ...
... Calorimetry is an accurate technique for determining enthalpy changes, but how do chemists deal with chemical systems that cannot be analyzed using this technique? For example, the rusting of iron (Figure 1) is extremely slow and, therefore, the resulting temperature change would be too small to be ...
Li−Fe−P−O2 Phase Diagram from First Principles Calculations
... reduced at µO2 ) -16.74 eV. Lower µO2 represents more reducing conditions, which generally correspond to higher temperatures and/or lower oxygen partial pressures and/or the presence of reducing agents. The predicted phase relations and reduction conditions compare well to experimental findings on s ...
... reduced at µO2 ) -16.74 eV. Lower µO2 represents more reducing conditions, which generally correspond to higher temperatures and/or lower oxygen partial pressures and/or the presence of reducing agents. The predicted phase relations and reduction conditions compare well to experimental findings on s ...
File
... (c) When iron is made into the alloy steel, the properties of iron are changed. High carbon steels are stronger than iron but are brittle. State a property of low carbon steels. ...
... (c) When iron is made into the alloy steel, the properties of iron are changed. High carbon steels are stronger than iron but are brittle. State a property of low carbon steels. ...
A* PLC Legacy GCSE Chemistry (all boards)
... There is reasonable accuracy that are oxidised and reduced. The spelling, punctuation in spelling, punctuation and The answer shows almost and grammar are very grammar, although there may faultless spelling, punctuation weak. still be some errors and grammar. Examples of chemistry points made in the ...
... There is reasonable accuracy that are oxidised and reduced. The spelling, punctuation in spelling, punctuation and The answer shows almost and grammar are very grammar, although there may faultless spelling, punctuation weak. still be some errors and grammar. Examples of chemistry points made in the ...
Chemical Kinetics
... conditions under which only one reactant varies significantly (pseudo first-order conditions). ...
... conditions under which only one reactant varies significantly (pseudo first-order conditions). ...
PHYSICAL SETTING CHEMISTRY
... (1) a greater quantity of charge and the same sign (2) a greater quantity of charge and the opposite sign (3) the same quantity of charge and the same sign (4) the same quantity of charge and the opposite sign 3 Which two notations represent atoms that are isotopes of the same element? ...
... (1) a greater quantity of charge and the same sign (2) a greater quantity of charge and the opposite sign (3) the same quantity of charge and the same sign (4) the same quantity of charge and the opposite sign 3 Which two notations represent atoms that are isotopes of the same element? ...
Basic Organic Chemistry Laboratory Course
... the chloroform solution gives a hint on the identity of the halogen present. Brown means that there is bromine, a violet colour points at iodine while chlorine is present if the colour of the solution does not change at all. If the iodine or the bromine test is positive, chlorine can be detected a ...
... the chloroform solution gives a hint on the identity of the halogen present. Brown means that there is bromine, a violet colour points at iodine while chlorine is present if the colour of the solution does not change at all. If the iodine or the bromine test is positive, chlorine can be detected a ...
chapter 18 - HCC Learning Web
... hydrogen in HCl is +1. To be reduced, the oxidation state of H must decrease. The obvious choice for the hydrogen product is H2(g), where hydrogen has a zero oxidation state. The balanced reaction is Mg(s) + 2HCl(aq) MgCl2(aq) + H2(g). Mg goes from the 0 to the +2 oxidation state by losing two ele ...
... hydrogen in HCl is +1. To be reduced, the oxidation state of H must decrease. The obvious choice for the hydrogen product is H2(g), where hydrogen has a zero oxidation state. The balanced reaction is Mg(s) + 2HCl(aq) MgCl2(aq) + H2(g). Mg goes from the 0 to the +2 oxidation state by losing two ele ...
Synthesis of Imidazolium Room-Temperature Ionic
... synthetic protocol for the synthesis of the ionic liquid allows not only the exploration of various reactions using ionic liquids as solvents (5, 6), but also the introduction of various concepts of green chemistry (7) and click chemistry (8). Green chemistry is based on principles that are designed ...
... synthetic protocol for the synthesis of the ionic liquid allows not only the exploration of various reactions using ionic liquids as solvents (5, 6), but also the introduction of various concepts of green chemistry (7) and click chemistry (8). Green chemistry is based on principles that are designed ...
WRL0001.tmp - Ethiopian Teachers Association
... Other complementary support to teachers include direct suggestions of instructional strategies to improve lessons., The strategies should challenge preconceptions and school-made misconceptions through recommending alternatives to the traditional approaches, such as setting up simplified laboratory ...
... Other complementary support to teachers include direct suggestions of instructional strategies to improve lessons., The strategies should challenge preconceptions and school-made misconceptions through recommending alternatives to the traditional approaches, such as setting up simplified laboratory ...
Energetics Past Paper Questions
... The lattice enthalpy of an ionic compound can be calculated using a Born-Haber cycle. Using lithium fluoride as the example, construct a Born-Haber cycle, labelling the cycle with the formulas and state symbols of the species present at each stage. (6) Two values of the lattice enthalpies for each o ...
... The lattice enthalpy of an ionic compound can be calculated using a Born-Haber cycle. Using lithium fluoride as the example, construct a Born-Haber cycle, labelling the cycle with the formulas and state symbols of the species present at each stage. (6) Two values of the lattice enthalpies for each o ...
OCR Document
... . Therefore 'pure' water is pH 5.67 rather than pH 7 because of dissolution of CO2. As pH of water increases due to presence of other bases, solubility of CO2 and total carbonate increases. . @ pH 4, log [CO32–] is about -5.0; @ pH 10, log [CO32–] ~–1.2. Ka1= [H+][HCO3–]/[H2CO3] = 4.45 X 10–7, Ka2 = ...
... . Therefore 'pure' water is pH 5.67 rather than pH 7 because of dissolution of CO2. As pH of water increases due to presence of other bases, solubility of CO2 and total carbonate increases. . @ pH 4, log [CO32–] is about -5.0; @ pH 10, log [CO32–] ~–1.2. Ka1= [H+][HCO3–]/[H2CO3] = 4.45 X 10–7, Ka2 = ...
Grossmont College Chemistry 141 Laboratory Manual 6th Edition
... a calorimeter or incomplete drying of a weighed precipitate), personal errors in reading an instrument or a measuring device (e.g. parallax error) or, biased methods implemented during the procedure (e.g. uncompensated human reaction times). Systematic errors often announce their presence in some so ...
... a calorimeter or incomplete drying of a weighed precipitate), personal errors in reading an instrument or a measuring device (e.g. parallax error) or, biased methods implemented during the procedure (e.g. uncompensated human reaction times). Systematic errors often announce their presence in some so ...
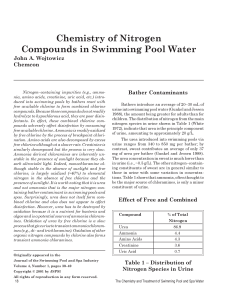
w_4-3 Chemistry of Nitrogen Compounds
... to chloroform), it will tend to volatilize into the atmosphere, thereby reducing the concentration in the water. This will be most pronounced in spas with their higher temperatures and use of aeration. Nitrogen trichloride is also decomposed by sunlight and it can undergo slow decomposition by hydro ...
... to chloroform), it will tend to volatilize into the atmosphere, thereby reducing the concentration in the water. This will be most pronounced in spas with their higher temperatures and use of aeration. Nitrogen trichloride is also decomposed by sunlight and it can undergo slow decomposition by hydro ...
Chemical Compounds
... 4. The oxidation state of hydrogen is generally +1 except when it is bonded to metals such as sodium (NaH) in which case it's oxidation number is -1. 5. Fluorine has an oxidation number of -1 in its compounds … always. Group 1 elements have an oxidation number of +1 in their compounds … always. Grou ...
... 4. The oxidation state of hydrogen is generally +1 except when it is bonded to metals such as sodium (NaH) in which case it's oxidation number is -1. 5. Fluorine has an oxidation number of -1 in its compounds … always. Group 1 elements have an oxidation number of +1 in their compounds … always. Grou ...
Entropy (Part I)
... The sign on ΔS for the system in a certain chemical change is negative. The ΔS value for the surroundings for the same reaction is positive. What would have to be true to make the reaction represent a spontaneous change? A. The ΔS of the system would have to have a greater magnitude than ΔS of th ...
... The sign on ΔS for the system in a certain chemical change is negative. The ΔS value for the surroundings for the same reaction is positive. What would have to be true to make the reaction represent a spontaneous change? A. The ΔS of the system would have to have a greater magnitude than ΔS of th ...
Redox

Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between species. The term ""redox"" comes from two concepts involved with electron transfer: reduction and oxidation. It can be explained in simple terms: Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.Although oxidation reactions are commonly associated with the formation of oxides from oxygen molecules, these are only specific examples of a more general concept of reactions involving electron transfer.Redox reactions, or oxidation-reduction reactions, have a number of similarities to acid–base reactions. Like acid–base reactions, redox reactions are a matched set, that is, there cannot be an oxidation reaction without a reduction reaction happening simultaneously. The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction. When writing half-reactions, the gained or lost electrons are typically included explicitly in order that the half-reaction be balanced with respect to electric charge.Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation and reduction properly refer to a change in oxidation state — the actual transfer of electrons may never occur. The oxidation state of an atom is the fictitious charge that an atom would have if all bonds between atoms of different elements were 100% ionic. Thus, oxidation is better defined as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classed as ""redox"" even though no electron transfer occurs (such as those involving covalent bonds).There are simple redox processes, such as the oxidation of carbon to yield carbon dioxide (CO2) or the reduction of carbon by hydrogen to yield methane (CH4), and more complex processes such as the oxidation of glucose (C6H12O6) in the human body through a series of complex electron transfer processes.