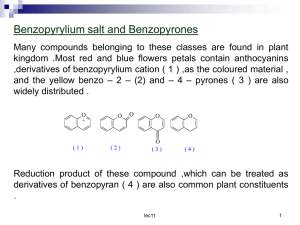
Stoichiometry
... Mole Ratio – the ratio of moles of one substance to moles of another substance in a balanced chemical equation The coefficients in a balanced equation give the relative numbers of molecules, as well as, the relative number of moles. ...
... Mole Ratio – the ratio of moles of one substance to moles of another substance in a balanced chemical equation The coefficients in a balanced equation give the relative numbers of molecules, as well as, the relative number of moles. ...
Lab Manual (Eng. Medium)
... barrel contains a little more than the required volume of liquid. Then the syringe is held with the needle pointed up and the plunger is pushed into eject the excess sample. Excess liquid is wiped off the needle with a tissue. Syringes should be cleaned immediately after use by rinsing them several ...
... barrel contains a little more than the required volume of liquid. Then the syringe is held with the needle pointed up and the plunger is pushed into eject the excess sample. Excess liquid is wiped off the needle with a tissue. Syringes should be cleaned immediately after use by rinsing them several ...
FREE Sample Here
... Topic: Section 6.3 Stoichiometry: The Arithmetic of Chemical Reactions Algo. Option: algorithmic 37) Balance the chemical equation given below, and determine the number of grams of MgO needed to produce 15.0 g of Fe2O3. ___ MgO(s) + ___ Fe(s) → ___ Fe2O3(s) + ___ Mg(s) A) 0.0877 g B) 1.26 g C) 3.78 ...
... Topic: Section 6.3 Stoichiometry: The Arithmetic of Chemical Reactions Algo. Option: algorithmic 37) Balance the chemical equation given below, and determine the number of grams of MgO needed to produce 15.0 g of Fe2O3. ___ MgO(s) + ___ Fe(s) → ___ Fe2O3(s) + ___ Mg(s) A) 0.0877 g B) 1.26 g C) 3.78 ...
Limiting Reactants and Percentage Yield
... Place the balls on an ungreased cookie sheet. 5. Bake at 350 °F for about 10 minutes, or until the cookies are light brown. ...
... Place the balls on an ungreased cookie sheet. 5. Bake at 350 °F for about 10 minutes, or until the cookies are light brown. ...
Section – B - About iTutoring
... ions of second group are less in comparison to solubility products of sulphides of metal of III B group ions, therefore, HCl is added before adding H2S water to test the second group ions. H2S(aq) 2H+(aq) + S2-(aq) HCl(aq) H+(aq) + Cl-(aq) The common ion available from HCl creates common ion effect ...
... ions of second group are less in comparison to solubility products of sulphides of metal of III B group ions, therefore, HCl is added before adding H2S water to test the second group ions. H2S(aq) 2H+(aq) + S2-(aq) HCl(aq) H+(aq) + Cl-(aq) The common ion available from HCl creates common ion effect ...
Concept based notes Chemistry Lab Manual
... ions from the dissociation of H2S gas combine with hydroxyl ions (OH-) from the dissociation of NH4OH to from nearly unionised H2O. The removal of H+ ions from the solution causes more H2S to dissociate, thereby increasing the concentration of S-2 ions to such an extent that ionic product of IV grou ...
... ions from the dissociation of H2S gas combine with hydroxyl ions (OH-) from the dissociation of NH4OH to from nearly unionised H2O. The removal of H+ ions from the solution causes more H2S to dissociate, thereby increasing the concentration of S-2 ions to such an extent that ionic product of IV grou ...
Chapter 5: Gases - HCC Learning Web
... 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 9. Calculate the amount of heat necessary to ...
... 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 9. Calculate the amount of heat necessary to ...
Homework 5-7 answers
... 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 9. Calculate the amount of heat necessary to ...
... 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 9. Calculate the amount of heat necessary to ...
File
... At room temperature and pressure chlorine does not behave as an ideal gas. At which temperature and pressure would the behaviour of chlorine become more ideal? ...
... At room temperature and pressure chlorine does not behave as an ideal gas. At which temperature and pressure would the behaviour of chlorine become more ideal? ...
SCH4U TEXT BOOK
... How do you predict whether or not a molecule that contains polar bonds has an overall molecular polarity? To determine molecular polarity, you must consider the shape of the molecule and the bond dipoles within the molecule. If equal bond dipoles act in opposite directions in three-dimensional space ...
... How do you predict whether or not a molecule that contains polar bonds has an overall molecular polarity? To determine molecular polarity, you must consider the shape of the molecule and the bond dipoles within the molecule. If equal bond dipoles act in opposite directions in three-dimensional space ...
Homework 5-8 answers
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
GCSE Chemistry Sample Assessment Materials
... The Sun is 72 % hydrogen and 26 % helium. The rest is made from other elements. Calculate the percentage of other elements in the Sun. ...
... The Sun is 72 % hydrogen and 26 % helium. The rest is made from other elements. Calculate the percentage of other elements in the Sun. ...
Chapter 1 Introduction to Forensic Chemistry
... Precipitation reactions begin with reagents dissolved in solution and produce a solid as a product. Combustion reactions involve the reaction of a substance with oxygen (often with applied heat); carbon-containing compounds then produce carbon dioxide and hydrogen-containing compounds then produce w ...
... Precipitation reactions begin with reagents dissolved in solution and produce a solid as a product. Combustion reactions involve the reaction of a substance with oxygen (often with applied heat); carbon-containing compounds then produce carbon dioxide and hydrogen-containing compounds then produce w ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... Enthalpy Change • We previously described processes as exothermic if they released heat, or endothermic if they absorbed heat. • The enthalpy of reaction is the amount of thermal energy that flows through a process. At constant pressure. DHrxn Tro's ―Introductory Chemistry‖, Chapter 8 ...
... Enthalpy Change • We previously described processes as exothermic if they released heat, or endothermic if they absorbed heat. • The enthalpy of reaction is the amount of thermal energy that flows through a process. At constant pressure. DHrxn Tro's ―Introductory Chemistry‖, Chapter 8 ...
Solubility and Complex-ion Equilibria
... • Fractional Precipitation • Fractional precipitation is the technique of separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another ion, and so forth. • The solubility of an insoluble salt can be manipulated by adding a species that reacts with e ...
... • Fractional Precipitation • Fractional precipitation is the technique of separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another ion, and so forth. • The solubility of an insoluble salt can be manipulated by adding a species that reacts with e ...
Experiments in General Chemistry: Featuring MeasureNet
... pictures and illustrations, all concept/technique experiments converted to a guided inquiry format, the addition of three new self-directed experiments, and one new Capstone experiment. Virtually all the artwork and illustrations, in the first edition of the manual, have been replaced with digital pi ...
... pictures and illustrations, all concept/technique experiments converted to a guided inquiry format, the addition of three new self-directed experiments, and one new Capstone experiment. Virtually all the artwork and illustrations, in the first edition of the manual, have been replaced with digital pi ...
Chemical Engineering Thermodynamics II
... A property is a macroscopic characteristic of a system such as pressure, temperature, volume, and mass. At a given state each property has a definite value independent of how the system arrived at that state. The properties of air in state 1 shown in Figure 1.1 are: pressure at 1 atm, temperature at ...
... A property is a macroscopic characteristic of a system such as pressure, temperature, volume, and mass. At a given state each property has a definite value independent of how the system arrived at that state. The properties of air in state 1 shown in Figure 1.1 are: pressure at 1 atm, temperature at ...
From Kinetics to Equilibrium
... that occur in vehicles: the decomposition of sodium azide in an air bag and the rusting of iron in steel. When an automobile collision activates an air bag, sodium azide, NaN3(g) , decomposes to form sodium, Na(s) , and nitrogen gas, N2(g). (The gas inflates the bag.) This chemical reaction occurs a ...
... that occur in vehicles: the decomposition of sodium azide in an air bag and the rusting of iron in steel. When an automobile collision activates an air bag, sodium azide, NaN3(g) , decomposes to form sodium, Na(s) , and nitrogen gas, N2(g). (The gas inflates the bag.) This chemical reaction occurs a ...
Equilibrium Reversible Reactions
... At this point, explain that in order for sodium chloride to break apart, heat is required. Place on the floor four pieces of red construction paper (to represent the heat), which can be picked up by the students representing the sodium chloride particles so that they can break up into sodium and chl ...
... At this point, explain that in order for sodium chloride to break apart, heat is required. Place on the floor four pieces of red construction paper (to represent the heat), which can be picked up by the students representing the sodium chloride particles so that they can break up into sodium and chl ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.