CP-Chem Ch 3 PowerPoint(Atomic Theory
... sample • Ex: Salt is NaCl, • it’s composition is always 39.4 % Na & 60.6% Cl ...
... sample • Ex: Salt is NaCl, • it’s composition is always 39.4 % Na & 60.6% Cl ...
HW Problems
... 10. Rubidium has two naturally occurring isotopes, rubidium-85 (mass 84.9118 amu, abundance 72.15%) and rubidium-87 (mass 86.9092 amu, abundance 27.85%). Calculate is the average atomic mass of rubidium. 11. Mass spectroscopy is a great technique for identifying molecular and atomic substances and s ...
... 10. Rubidium has two naturally occurring isotopes, rubidium-85 (mass 84.9118 amu, abundance 72.15%) and rubidium-87 (mass 86.9092 amu, abundance 27.85%). Calculate is the average atomic mass of rubidium. 11. Mass spectroscopy is a great technique for identifying molecular and atomic substances and s ...
Structure of Atoms
... • The atomic number equals the number of protons.The mass number equals the total number of subatomic particles in the nucleus. – atomic number: the number of protons in the nucleus of an atom – mass number: the sum of the numbers of protons and neutrons in the nucleus of an atom ...
... • The atomic number equals the number of protons.The mass number equals the total number of subatomic particles in the nucleus. – atomic number: the number of protons in the nucleus of an atom – mass number: the sum of the numbers of protons and neutrons in the nucleus of an atom ...
Chapter 4 - Mr. Fischer.com
... Defining the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. A. Early philosophers believed that atoms were indivisible and indestructible. B. Dalton’s Atomic theory. Dalton used experimental methods, to transform Democritus’s ideas on atoms into ...
... Defining the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. A. Early philosophers believed that atoms were indivisible and indestructible. B. Dalton’s Atomic theory. Dalton used experimental methods, to transform Democritus’s ideas on atoms into ...
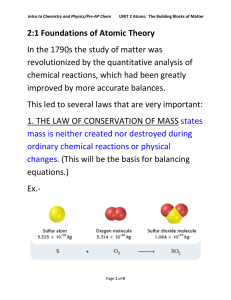
2:1 Foundations of Atomic Theory In the 1790s the study of matter
... are 2000 times larger than an electron. If a large football stadium were an atom, this would make the nucleus (including the protons and neutrons) about the size of a marble. The electrons would be similar to dust particles floating about the stadium. This means that in our current model of the atom ...
... are 2000 times larger than an electron. If a large football stadium were an atom, this would make the nucleus (including the protons and neutrons) about the size of a marble. The electrons would be similar to dust particles floating about the stadium. This means that in our current model of the atom ...
Organic Chemistry - Ms. Chambers' Biology
... (genetic information) DNA (deoxyribonucleic acid) and RNA Monomer: nucleotide ...
... (genetic information) DNA (deoxyribonucleic acid) and RNA Monomer: nucleotide ...
CHAPTER 3, ATOMS: THE BUILDING BLOCKS OF MATTER
... during ordinary chemical reactions or physical changes. The law of definite proportions states that a chemical compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound. The law of multiple proportions states that if tw ...
... during ordinary chemical reactions or physical changes. The law of definite proportions states that a chemical compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound. The law of multiple proportions states that if tw ...
Chapter 4 Notes
... Due to differences in mass (# of neutrons) the paths of the molecules curve based on their individual mass. Heavier particles curve less. This change in curvature causes the particles to land on different places on a detector. Draw picture of mass spectrometer below The mass spectrometer was invente ...
... Due to differences in mass (# of neutrons) the paths of the molecules curve based on their individual mass. Heavier particles curve less. This change in curvature causes the particles to land on different places on a detector. Draw picture of mass spectrometer below The mass spectrometer was invente ...
Chapter 4: The Chemical Basis of Life
... o Forms by the attraction of the oily parts of lipid molecules for each other and by the attraction of the other parts of the lipid molecules for the surrounding water ...
... o Forms by the attraction of the oily parts of lipid molecules for each other and by the attraction of the other parts of the lipid molecules for the surrounding water ...
Topic 2 Part 1 Slides - Coral Gables Senior High
... molecules and compounds” True! Atoms chemically combine together in whole-number ratios to form compounds and molecules. i.e. H₂O and CO₂ ...
... molecules and compounds” True! Atoms chemically combine together in whole-number ratios to form compounds and molecules. i.e. H₂O and CO₂ ...
PS7aChemistryReviewRevised
... Instant coffee dissolves in water. Chocolate melts in a warm room ...
... Instant coffee dissolves in water. Chocolate melts in a warm room ...
Foldable - Georgetown ISD
... Practice: How many electrons, protons, and neutrons are present in Potassium-39? ...
... Practice: How many electrons, protons, and neutrons are present in Potassium-39? ...
Page 1
... 3. Aristotle thought that all matter was built up from only four elements. These elements were earth, air, fire, and water 4. Dalton suggested that compounds have a fixed composition. Explain that statement. No matter how large or small the sample, the ratio of the masses in the compound is always t ...
... 3. Aristotle thought that all matter was built up from only four elements. These elements were earth, air, fire, and water 4. Dalton suggested that compounds have a fixed composition. Explain that statement. No matter how large or small the sample, the ratio of the masses in the compound is always t ...
Chemical Reactions
... • Synthesis – 2 substances (reactants) combine to form a new substance (product). – Substances are either atoms (elements) or compounds in this case. A + ...
... • Synthesis – 2 substances (reactants) combine to form a new substance (product). – Substances are either atoms (elements) or compounds in this case. A + ...
File
... • The atomic number, Z, represents the number of protons, p+, in the nucleus of an atom. • The atomic number is usually the biggest number listed in the box for each element (look at periodic table). • The atomic number (or number of protons) identifies an element. • The modern periodic table orders ...
... • The atomic number, Z, represents the number of protons, p+, in the nucleus of an atom. • The atomic number is usually the biggest number listed in the box for each element (look at periodic table). • The atomic number (or number of protons) identifies an element. • The modern periodic table orders ...
BASIC CHEMISTRY
... The atomic number for O is 8. How many protons in O? How many electrons in O? The atomic mass of O is 16. How many neutrons in O? Draw an Oxygen atom. Show the number of protons and neutrons in the nucleus and the electrons in the energy ...
... The atomic number for O is 8. How many protons in O? How many electrons in O? The atomic mass of O is 16. How many neutrons in O? Draw an Oxygen atom. Show the number of protons and neutrons in the nucleus and the electrons in the energy ...
Test 1 - UTC.edu
... 14. Which one of the following statements about atoms and subatomic particles is correct? A) The proton and the neutron have identical masses. B) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons C) The neutron's mass is equal to that of a proton plus an electron. D) An ...
... 14. Which one of the following statements about atoms and subatomic particles is correct? A) The proton and the neutron have identical masses. B) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons C) The neutron's mass is equal to that of a proton plus an electron. D) An ...
Development of the Atomic Theory
... Mass Matter, like energy, is neither created nor destroyed in an ordinary chemical reaction “One may take it for granted that in every reaction there is an equal quantity of matter before and after.” ...
... Mass Matter, like energy, is neither created nor destroyed in an ordinary chemical reaction “One may take it for granted that in every reaction there is an equal quantity of matter before and after.” ...
11/13 atoms powerpoint
... All matter is composed of extremely small particles called atoms Atoms of a given element are identical in size, mass, and other properties; atoms of different John Dalton elements differ in size, mass, and other properties Atoms cannot be subdivided, created, or destroyed Atoms of different ...
... All matter is composed of extremely small particles called atoms Atoms of a given element are identical in size, mass, and other properties; atoms of different John Dalton elements differ in size, mass, and other properties Atoms cannot be subdivided, created, or destroyed Atoms of different ...
CHEMISTRY AND ORGANIC MOLECULES Matter: Has mass and
... Protons and neutrons have same mass (about 2000X that of electron) So electrons really don’t figure into atomic mass Proton, electron equal, but opposite, charges Protons, neutrons equal masses Atomic Number: at lower left of atomic symbol = number of protons Atomic Weight: protons plus neutrons Iso ...
... Protons and neutrons have same mass (about 2000X that of electron) So electrons really don’t figure into atomic mass Proton, electron equal, but opposite, charges Protons, neutrons equal masses Atomic Number: at lower left of atomic symbol = number of protons Atomic Weight: protons plus neutrons Iso ...
General Biology I Online – Lab Midterm REVIEW
... What are endergonic and exergonic reactions? What are biological catalysts? What do catalysts interact with? What is the lock and key fit? What is ATP? What is metabolism? What is anabolic and catabolic? Most enzymes are what? What are the two laws of Thermodynamics? What is the formula for cellular ...
... What are endergonic and exergonic reactions? What are biological catalysts? What do catalysts interact with? What is the lock and key fit? What is ATP? What is metabolism? What is anabolic and catabolic? Most enzymes are what? What are the two laws of Thermodynamics? What is the formula for cellular ...
General Biology I Online – Lecture Midterm REVIEW (2).
... What are endergonic and exergonic reactions? What are biological catalysts? What do catalysts interact with? What is the lock and key fit? What is ATP? What is metabolism? What is anabolic and catabolic? Most enzymes are what? What are the two laws of Thermodynamics? What is the formula for cellular ...
... What are endergonic and exergonic reactions? What are biological catalysts? What do catalysts interact with? What is the lock and key fit? What is ATP? What is metabolism? What is anabolic and catabolic? Most enzymes are what? What are the two laws of Thermodynamics? What is the formula for cellular ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.