chapter 4 types of chemical reactions and solution stoichiometry
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
... unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges as in ionic compounds but are charges much smaller in magnitude. Water is a polar molecule and ...
Chem Soc Rev
... resource, a major component of natural gas, coal-bed gas and shale gas, but also from a variety of renewable sources as biogas,14 could provide an economical and sustainable alternative to petroleum. Furthermore, methane is one of the most destructive greenhouse gas. Thus, the transformation of meth ...
... resource, a major component of natural gas, coal-bed gas and shale gas, but also from a variety of renewable sources as biogas,14 could provide an economical and sustainable alternative to petroleum. Furthermore, methane is one of the most destructive greenhouse gas. Thus, the transformation of meth ...
Catalytic oxidation of ammonia to nitrogen
... The emissions of nitrogen oxides (NOx) and sulphur oxides (SOx) give rise to acidification of the environment. NOx and SOx are converted in the atmosphere to give nitric and sulphuric acid. However emission of ammonia causes acidification of the environment in an indirect way. Reaction of ammonia wi ...
... The emissions of nitrogen oxides (NOx) and sulphur oxides (SOx) give rise to acidification of the environment. NOx and SOx are converted in the atmosphere to give nitric and sulphuric acid. However emission of ammonia causes acidification of the environment in an indirect way. Reaction of ammonia wi ...
Corrosion of Ceramic and Composite Materials, Second Edition
... liquid phase sintering (also crystal growth studies) and the dissolution of various raw materials in molten glass in the manufacture of glass products. The proper selection of materials and good design practices can greatly reduce the cost caused by corrosion. To make the proper selection, engineers ...
... liquid phase sintering (also crystal growth studies) and the dissolution of various raw materials in molten glass in the manufacture of glass products. The proper selection of materials and good design practices can greatly reduce the cost caused by corrosion. To make the proper selection, engineers ...
Chemical Equilibrium - local.brookings.k12.sd.us
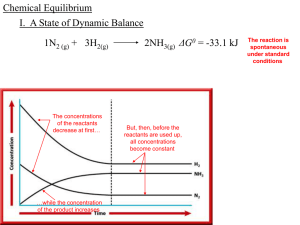
... I. A State of Dynamic Balance -when a ________ reaction results in the almost ________ complete conversion of ________ reactants to ________, products the 1N2 (g) + 3H2(g) 2NH3(g) ________ reaction is said to go to completion but _____ most _________ reactions __________, 1N2 (g) + 3H2(g) 2NH3(g) __ ...
... I. A State of Dynamic Balance -when a ________ reaction results in the almost ________ complete conversion of ________ reactants to ________, products the 1N2 (g) + 3H2(g) 2NH3(g) ________ reaction is said to go to completion but _____ most _________ reactions __________, 1N2 (g) + 3H2(g) 2NH3(g) __ ...
chapter 5 gases and the kinetic
... a) As the pressure on a gas increases, the molecules move closer together, decreasing the volume. When the pressure is tripled, the volume decreases to one third of the original volume at constant temperature (Boyle’s Law). b) As the temperature of a gas increases, the gas molecules gain kinetic ene ...
... a) As the pressure on a gas increases, the molecules move closer together, decreasing the volume. When the pressure is tripled, the volume decreases to one third of the original volume at constant temperature (Boyle’s Law). b) As the temperature of a gas increases, the gas molecules gain kinetic ene ...
Appendices
... contains 26.76% C, 2.21% H, 71.17% O and has a molar mass of 90.04 g/mol. Determine the molecular formula for this substance. 30. Eucalyptus leaves are the food source for panda bears. Eucalyptol is an oil found in these leaves. Analysis of eucalyptol indicates it has a molar mass of 154 g/mol and c ...
... contains 26.76% C, 2.21% H, 71.17% O and has a molar mass of 90.04 g/mol. Determine the molecular formula for this substance. 30. Eucalyptus leaves are the food source for panda bears. Eucalyptol is an oil found in these leaves. Analysis of eucalyptol indicates it has a molar mass of 154 g/mol and c ...
The chemistry of beer aging – a critical review Food Chemistry
... generalization of the sensory evolution during beer storage and is by no means applicable to every beer. A constant decrease in bitterness is observed during aging. This is partly due to sensory masking by an increasing sweet taste. In contrast to an initial acceleration of sweet aroma development, ...
... generalization of the sensory evolution during beer storage and is by no means applicable to every beer. A constant decrease in bitterness is observed during aging. This is partly due to sensory masking by an increasing sweet taste. In contrast to an initial acceleration of sweet aroma development, ...
volume 2 - PianetaChimica
... the aim was not only to make use of past recordings but also to give them such a form that they may be used in practice and further chemical education. Consequently, it was necessary to make some corrections in order to unify the form of the problems. However, they did not concern the contents and l ...
... the aim was not only to make use of past recordings but also to give them such a form that they may be used in practice and further chemical education. Consequently, it was necessary to make some corrections in order to unify the form of the problems. However, they did not concern the contents and l ...
Kinetic investigation of low-pH Fe(II) oxidation and development of a
... emitted from environmental facilities and industrial processes such as petroleum refineries, paper manufacturing, anaerobic digestion processes etc. The removal of H2S from gaseous streams is required for the health of the general public, occupational safety and for operational reasons. The Liquid R ...
... emitted from environmental facilities and industrial processes such as petroleum refineries, paper manufacturing, anaerobic digestion processes etc. The removal of H2S from gaseous streams is required for the health of the general public, occupational safety and for operational reasons. The Liquid R ...
College Chemistry
... measurement were exact to the nearest 0.01 cm, it would have been recorded as 15.70 cm. We say that the first measurement is accurate to 3 significant figures and the second to 4. A recorded volume of 2.8 L represents two significant figures. If this same volume were written 0.028 m3, it would still ...
... measurement were exact to the nearest 0.01 cm, it would have been recorded as 15.70 cm. We say that the first measurement is accurate to 3 significant figures and the second to 4. A recorded volume of 2.8 L represents two significant figures. If this same volume were written 0.028 m3, it would still ...
Chemical Redox Agents for Organometallic
... cells,7a,b which are very limited in the quantities of reagent that can be produced, preparative electrochemical cells have reaction times of tens of minutes, a time frame that may be troublesome if the desired product has limited stability. Homogeneous chemical redox reactions, on the other hand, o ...
... cells,7a,b which are very limited in the quantities of reagent that can be produced, preparative electrochemical cells have reaction times of tens of minutes, a time frame that may be troublesome if the desired product has limited stability. Homogeneous chemical redox reactions, on the other hand, o ...
Chemical Redox Agents for Organometallic
... cells,7a,b which are very limited in the quantities of reagent that can be produced, preparative electrochemical cells have reaction times of tens of minutes, a time frame that may be troublesome if the desired product has limited stability. Homogeneous chemical redox reactions, on the other hand, o ...
... cells,7a,b which are very limited in the quantities of reagent that can be produced, preparative electrochemical cells have reaction times of tens of minutes, a time frame that may be troublesome if the desired product has limited stability. Homogeneous chemical redox reactions, on the other hand, o ...
edexcel_u4_2010_2013..
... 20 At 100 °C, pure water has a pH of 6, whereas at 25 °C it has a pH of 7. This is because A ...
... 20 At 100 °C, pure water has a pH of 6, whereas at 25 °C it has a pH of 7. This is because A ...
1 Ag PO 7.5 10 1.79 10 418.57 mol x gL x M g
... enough that HgS does precipitate. First, we calculate the highest pH at which FeS will remain soluble, by using Kspa for FeS. (Recall that a saturated solution of H2S = 0.10 M.) ...
... enough that HgS does precipitate. First, we calculate the highest pH at which FeS will remain soluble, by using Kspa for FeS. (Recall that a saturated solution of H2S = 0.10 M.) ...
Metallocene Organoactinide Complexes
... In this particular review we will provide an overview of the preparation and properties of the major classes of actinide complexes containing different cyclopentadienyl ligands. Discussions are classified on the basis of formal oxidation states and we are confining our discussions only to the oxidatio ...
... In this particular review we will provide an overview of the preparation and properties of the major classes of actinide complexes containing different cyclopentadienyl ligands. Discussions are classified on the basis of formal oxidation states and we are confining our discussions only to the oxidatio ...
volume 2 - HotNews
... the aim was not only to make use of past recordings but also to give them such a form that they may be used in practice and further chemical education. Consequently, it was necessary to make some corrections in order to unify the form of the problems. However, they did not concern the contents and l ...
... the aim was not only to make use of past recordings but also to give them such a form that they may be used in practice and further chemical education. Consequently, it was necessary to make some corrections in order to unify the form of the problems. However, they did not concern the contents and l ...
AQA Science GCSE Chemistry
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
Sample Chapter 3
... • The mole maintains the same numerical relationship between mass on the atomic scale (atomic mass units, amu) and mass on the macroscopic scale (grams, g). In everyday terms, a grocer does not know that there are 1 dozen eggs from their weight or that there is 1 kilogram of coffee beans from their ...
... • The mole maintains the same numerical relationship between mass on the atomic scale (atomic mass units, amu) and mass on the macroscopic scale (grams, g). In everyday terms, a grocer does not know that there are 1 dozen eggs from their weight or that there is 1 kilogram of coffee beans from their ...
Section 1
... We can picture the hydrogen atom — the simplest of all atoms with one electron and one proton in the nucleus — by considering a pea placed in the centre of a football pitch, to represent the nucleus with its proton. On this scale the electron will revolve in a circular orbit round the goalposts. Bet ...
... We can picture the hydrogen atom — the simplest of all atoms with one electron and one proton in the nucleus — by considering a pea placed in the centre of a football pitch, to represent the nucleus with its proton. On this scale the electron will revolve in a circular orbit round the goalposts. Bet ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.