Entropy (Part I)
... • Any process that occurs without outside intervention is spontaneous. • When an egg is dropped, it spontaneously breaks. • The reverse reaction is not spontaneous. (Humpty Dumpty couldnʼt be put back together again.) • A spontaneous process therefore occurs in only one direction. (At a given ...
... • Any process that occurs without outside intervention is spontaneous. • When an egg is dropped, it spontaneously breaks. • The reverse reaction is not spontaneous. (Humpty Dumpty couldnʼt be put back together again.) • A spontaneous process therefore occurs in only one direction. (At a given ...
Physical and Chemical Changes
... • A substance is changed physically, but not chemically. It is still the same substance. • Ex: Ice melts into water. It is still H2O, just in a different physical form. ...
... • A substance is changed physically, but not chemically. It is still the same substance. • Ex: Ice melts into water. It is still H2O, just in a different physical form. ...
Equilibrium Constant- Keq
... c) Calculate the equilibrium constant d) Describe the percent reaction. 4. Hydrogen Chloride is produced from hydrogen and chlorine gases. At equilibrium, the hydrogen concentration is 0.12 mol/L and chlorine is 0.10 mol/L. Find the concentration of the hydrogen chloride if the equilibrium constant ...
... c) Calculate the equilibrium constant d) Describe the percent reaction. 4. Hydrogen Chloride is produced from hydrogen and chlorine gases. At equilibrium, the hydrogen concentration is 0.12 mol/L and chlorine is 0.10 mol/L. Find the concentration of the hydrogen chloride if the equilibrium constant ...
chemical*equations
... When'a'chemical'reaction'occurs,' atoms'rearrange'to'form'new' compounds,'but'no'new'atoms'are' created'nor'are'any'destroyed.'This' concept'is'called'conservation'of' mass.'Mass'conservation'can'be' seen'in'a'balanced'chemical' equation,'where'the'numbers'of' each'kind'of'atom'are'the'same'on' both ...
... When'a'chemical'reaction'occurs,' atoms'rearrange'to'form'new' compounds,'but'no'new'atoms'are' created'nor'are'any'destroyed.'This' concept'is'called'conservation'of' mass.'Mass'conservation'can'be' seen'in'a'balanced'chemical' equation,'where'the'numbers'of' each'kind'of'atom'are'the'same'on' both ...
Chemistry Standard Course of Study -- Detailed - UNCG GK-12
... BP, brittle, and high electrical conductivity either in molten state or in aqueous solution. Explain how covalent bonding in compounds determines their characteristics: low MP, low BP, poor electrical conductivity, polar nature, etc. Describe metallic bonds: “metal ions plus ‘sea’ of mobile elec ...
... BP, brittle, and high electrical conductivity either in molten state or in aqueous solution. Explain how covalent bonding in compounds determines their characteristics: low MP, low BP, poor electrical conductivity, polar nature, etc. Describe metallic bonds: “metal ions plus ‘sea’ of mobile elec ...
Chemical Reactions Practice Test
... 1. If 3408 grams of C2H6 are reacted and 5301 grams of water are actually produced, what is the percent yield? ...
... 1. If 3408 grams of C2H6 are reacted and 5301 grams of water are actually produced, what is the percent yield? ...
- Gondwana University, Gadchiroli
... capacity at constant volume & at constant pressure, Joule-Thomson experiment, Joule Thomson coefficient & Inversion temperature, calculations of W,Q,ΔE & ΔH for expansion of gases for isothermal & adiabatic conditions for reversible process, carnot’s cycle & its efficiency, thermodynamic scale of te ...
... capacity at constant volume & at constant pressure, Joule-Thomson experiment, Joule Thomson coefficient & Inversion temperature, calculations of W,Q,ΔE & ΔH for expansion of gases for isothermal & adiabatic conditions for reversible process, carnot’s cycle & its efficiency, thermodynamic scale of te ...
Elements, Compounds and Chemical Reactions
... then silicon, and our bodies are oxygen and then carbon. ...
... then silicon, and our bodies are oxygen and then carbon. ...
L1 – CHEMISTRY FINAL REVIEW
... c. HBr- Polar molecule; dipole-dipole interactions between the molecules; medium strength interactions. 28. What are some major differences between the following types of solids? In terms of Mpt/Bpt, solubility, and conductivity. Metallic, Ionic, Polar Molecular, and Nonpolar Molecular. Give an exam ...
... c. HBr- Polar molecule; dipole-dipole interactions between the molecules; medium strength interactions. 28. What are some major differences between the following types of solids? In terms of Mpt/Bpt, solubility, and conductivity. Metallic, Ionic, Polar Molecular, and Nonpolar Molecular. Give an exam ...
FREE Sample Here
... 78) How would the lack of a cofactor for an enzyme affect that enzyme's function? A) The enzyme would cease to function after reaching a maximum rate. B) The enzyme would function more slowly. C) The enzyme's function would not be altered. D) The enzyme would not be able to function. E) The enzyme w ...
... 78) How would the lack of a cofactor for an enzyme affect that enzyme's function? A) The enzyme would cease to function after reaching a maximum rate. B) The enzyme would function more slowly. C) The enzyme's function would not be altered. D) The enzyme would not be able to function. E) The enzyme w ...
View PDF
... ____ 17. In the reaction A + B → C + D, if the quantity of B is insufficient to react with all of A, a. A is the limiting reactant. c. there is no limiting reactant. b. B is the limiting reactant. d. no product can be formed. ____ 18. What is the maximum possible amount of product obtained in a che ...
... ____ 17. In the reaction A + B → C + D, if the quantity of B is insufficient to react with all of A, a. A is the limiting reactant. c. there is no limiting reactant. b. B is the limiting reactant. d. no product can be formed. ____ 18. What is the maximum possible amount of product obtained in a che ...
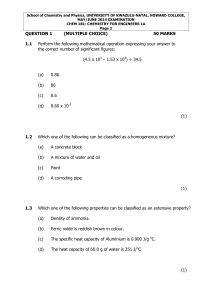
School of Chemistry
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
2010 Exam
... Select the letter of the correct response from those provided. EITHER shade the letter on your computer scorable card OR place the letter in the blank provided on your Multiple Choice Answer Sheet, whichever format is being used by your school for this exam. Do ALL questions in this section. ...
... Select the letter of the correct response from those provided. EITHER shade the letter on your computer scorable card OR place the letter in the blank provided on your Multiple Choice Answer Sheet, whichever format is being used by your school for this exam. Do ALL questions in this section. ...
A Level Chemistry.pub
... Change to all A Levels Changes are under way for all A levels in all schools and colleges and some awarding bodies are still revising their syllabuses for 2015. As a result, this guide is an illustration of the content but the exact details may change. The most significant changes in A Levels and AS ...
... Change to all A Levels Changes are under way for all A levels in all schools and colleges and some awarding bodies are still revising their syllabuses for 2015. As a result, this guide is an illustration of the content but the exact details may change. The most significant changes in A Levels and AS ...
Gas and Thermo Notes
... Heat - the energy that is transferred from one object to another because of a difference of temperature. Temperature - the measure of hotness or coldness relative to a standard, it is related to the kinetic energy of the molecules in that substance.. Temperature is a measure of the direction heat wi ...
... Heat - the energy that is transferred from one object to another because of a difference of temperature. Temperature - the measure of hotness or coldness relative to a standard, it is related to the kinetic energy of the molecules in that substance.. Temperature is a measure of the direction heat wi ...
Document
... When a solution of sodium sulfate and barium chloride are mixed, the sulfate anion combines with the barium cation and this combination precipitates as barium sulfate. The newly formed sodium chloride remains soluble. ...
... When a solution of sodium sulfate and barium chloride are mixed, the sulfate anion combines with the barium cation and this combination precipitates as barium sulfate. The newly formed sodium chloride remains soluble. ...
Section 7.1 Describing Reactions
... you expect to learn. After reading, state what you learned about each item you listed. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the end of your textbook. What I Expect to Learn ...
... you expect to learn. After reading, state what you learned about each item you listed. For more information on this Reading Strategy, see the Reading and Study Skills in the Skills and Reference Handbook at the end of your textbook. What I Expect to Learn ...
Chapter 3 – Stoichiometry of Formulas and Equations This chapter
... CaCO3 CaO + CO2 (with heating) Reactions that Involve a Limiting Reagent In practice, reactions are rarely conducted with exact stoichiometric amounts of each reagent for three reasons. First, if one of the reactants is a gas, it may be difficult to measure out that reactant. Second, one reactant ...
... CaCO3 CaO + CO2 (with heating) Reactions that Involve a Limiting Reagent In practice, reactions are rarely conducted with exact stoichiometric amounts of each reagent for three reasons. First, if one of the reactants is a gas, it may be difficult to measure out that reactant. Second, one reactant ...
NSCC Chem 121 chapter5
... the numerical value and units of the quantity. • Step 2: Leave some working space and set the known quantity equal to the units of the unknown quantity. • Step 3: Multiply the known quantity by one or more factors, such that the units of the factor cancel the units of the known quantity and generate ...
... the numerical value and units of the quantity. • Step 2: Leave some working space and set the known quantity equal to the units of the unknown quantity. • Step 3: Multiply the known quantity by one or more factors, such that the units of the factor cancel the units of the known quantity and generate ...
unit 7 – writing and balancing chemical equations
... side of the equation) by adding a coefficient in front of the entire compound. (not in the middle of the compound) (6) If the polyatomic comes apart OR if there is no polyatomic present, balance the non-metals except hydrogen and oxygen next by adding coefficients where necessary (7) Balance the hyd ...
... side of the equation) by adding a coefficient in front of the entire compound. (not in the middle of the compound) (6) If the polyatomic comes apart OR if there is no polyatomic present, balance the non-metals except hydrogen and oxygen next by adding coefficients where necessary (7) Balance the hyd ...
Test 1 Pre test
... All reactant collisions result in product formation. Activation energy is always positive. Reactant molecules must absorb energy to form the transition state. Reactant collisions must be oriented properly to form products. ...
... All reactant collisions result in product formation. Activation energy is always positive. Reactant molecules must absorb energy to form the transition state. Reactant collisions must be oriented properly to form products. ...
1. Review (MC problems, due Monday) 2. - mvhs
... following it. Select the one lettered choice that best fits each statement. A choice may be used once, more than once, or not at all. (A) An ionic solid (B) A metallic solid (C) A network solid with covalent bonds (D) A molecular solid with hydrogen bonding (E) A molecular solid with nonpolar molecu ...
... following it. Select the one lettered choice that best fits each statement. A choice may be used once, more than once, or not at all. (A) An ionic solid (B) A metallic solid (C) A network solid with covalent bonds (D) A molecular solid with hydrogen bonding (E) A molecular solid with nonpolar molecu ...
Ch 11 Chemical Reactions
... Assemble the correct formulas for all the reactants and products, using “+” and “→” Count the atoms of each type appearing on both sides Treat polyatomic ions like an “element” if they are unchanged by the reaction Balance the elements one at a time by adding coefficients (the numbers in front) wher ...
... Assemble the correct formulas for all the reactants and products, using “+” and “→” Count the atoms of each type appearing on both sides Treat polyatomic ions like an “element” if they are unchanged by the reaction Balance the elements one at a time by adding coefficients (the numbers in front) wher ...
Bioorthogonal chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical reporter) and introduced to the cell; substrates include metabolites, enzyme inhibitors, etc. The chemical reporter must not alter the structure of the substrate dramatically to avoid affecting its bioactivity. Secondly, a probe containing the complementary functional group is introduced to react and label the substrate.Although effective bioorthogonal reactions such as copper-free click chemistry have been developed, development of new reactions continues to generate orthogonal methods for labeling to allow multiple methods of labeling to be used in the same biosystems.