Lecture 15b
... their ability to form hydrogen bonds with water. • Most ethers react with oxygen in air in the presence of light to form explosive peroxides, which have higher boiling points that the ethers themselves. • Diethyl ether and tetrahydrofuran are often inhibited with BHT (3,5-di-tert.-butyl-4-hydroxytol ...
... their ability to form hydrogen bonds with water. • Most ethers react with oxygen in air in the presence of light to form explosive peroxides, which have higher boiling points that the ethers themselves. • Diethyl ether and tetrahydrofuran are often inhibited with BHT (3,5-di-tert.-butyl-4-hydroxytol ...
Lecture 4
... • Chloramines are formed by combining ammonia (NH3) and chlorine (Cl2) • Chloramines are less reactive than chlorine – Not as strong a disinfectant as chlorine – Form less DBPs – Persist in distribution system longer, thus can be more effective against biofilms ...
... • Chloramines are formed by combining ammonia (NH3) and chlorine (Cl2) • Chloramines are less reactive than chlorine – Not as strong a disinfectant as chlorine – Form less DBPs – Persist in distribution system longer, thus can be more effective against biofilms ...
Chemistry 20 Lesson 36 – The Whole Enchilada
... Compare the volume that 0.278 mol of hydrogen would occupy at STP and SATP. ...
... Compare the volume that 0.278 mol of hydrogen would occupy at STP and SATP. ...
Contents
... Biosynthesis of the Nucleotide Loop of Cobalamins: Conversion of Cobyric Acid to Cobalamin, 148 ...
... Biosynthesis of the Nucleotide Loop of Cobalamins: Conversion of Cobyric Acid to Cobalamin, 148 ...
W19 Aldehydes ketones I
... nucleophilic addition AN to C=O group: cyanohydrins, hemiacetals, acetals, thioacetals nucleophilic addition-elimination AN(E) to C=O group: imines, oximes, hydrazones, enamines nucleophilic addition of phosphorus ylides oxidation of aldehydes and ketones ...
... nucleophilic addition AN to C=O group: cyanohydrins, hemiacetals, acetals, thioacetals nucleophilic addition-elimination AN(E) to C=O group: imines, oximes, hydrazones, enamines nucleophilic addition of phosphorus ylides oxidation of aldehydes and ketones ...
resonance effects - HCC Learning Web
... Reaction with a mixture of sulfuric acid and SO3 Reactive species is sulfur trioxide or its conjugate ...
... Reaction with a mixture of sulfuric acid and SO3 Reactive species is sulfur trioxide or its conjugate ...
Energy and Matter in Chemical Change Science 10
... because both the water pressure and the opening of a faucet have an impact on how much water flows. If we change both of them at the same time, we can't be sure how much of the change in water flow is because of the faucet opening and how much because of the water pressure. In other words, it would ...
... because both the water pressure and the opening of a faucet have an impact on how much water flows. If we change both of them at the same time, we can't be sure how much of the change in water flow is because of the faucet opening and how much because of the water pressure. In other words, it would ...
practice exercise - Needham.K12.ma.us
... hydroxide. (c) You must determine the charge of Fe in this compound because an iron atom can form more than one cation. Because the compound contains three Cl– ions, the cation must be Fe3+ which is the iron(III), or ferric, ion. The Cl– ion is the chloride ion. Thus, the compound is iron(III) chlor ...
... hydroxide. (c) You must determine the charge of Fe in this compound because an iron atom can form more than one cation. Because the compound contains three Cl– ions, the cation must be Fe3+ which is the iron(III), or ferric, ion. The Cl– ion is the chloride ion. Thus, the compound is iron(III) chlor ...
organic chemistry i
... Isomer number and tetrahedral carbon Optical activity. Plane-polarized light The polarimeter Specific rotation Enantionmerism: the discovery Enantiomerism and tetrahedral carbon Enantiomerism and optical activity Prediction of enantiomerism. Chirality The chiral center Enantiomers The racemic modifi ...
... Isomer number and tetrahedral carbon Optical activity. Plane-polarized light The polarimeter Specific rotation Enantionmerism: the discovery Enantiomerism and tetrahedral carbon Enantiomerism and optical activity Prediction of enantiomerism. Chirality The chiral center Enantiomers The racemic modifi ...
1. Natures Chemistry Unit Questions
... o Aldehydes can pack reasonable closely together o Ketones aren’t as straight and cant pack as well o Like proteins the intermolecular forces are broken on heating and this changes the taste and texture of molecules o Aldehydes and ketones are relatively soluble so can be washed out of foods during ...
... o Aldehydes can pack reasonable closely together o Ketones aren’t as straight and cant pack as well o Like proteins the intermolecular forces are broken on heating and this changes the taste and texture of molecules o Aldehydes and ketones are relatively soluble so can be washed out of foods during ...
Chapter 4 - Nomenclature and Conformations of Alkanes and
... - Cycloalkanes are alkanes in which all or some of the carbon atoms are arranged in a ring - Alkanes have the general formula of Cn H2n+2 - Cycloalkanes with a single ring have the formula Cn H2n 4.2 - Shapes of Alkanes - An unbranched chain means that each carbon atom is bonded to no more than two ...
... - Cycloalkanes are alkanes in which all or some of the carbon atoms are arranged in a ring - Alkanes have the general formula of Cn H2n+2 - Cycloalkanes with a single ring have the formula Cn H2n 4.2 - Shapes of Alkanes - An unbranched chain means that each carbon atom is bonded to no more than two ...
Topics 10 and 20 Outline
... Essential idea: Organic chemistry focuses on the chemistry of compounds containing carbon. Nature of science: Serendipity and scientific discoveries—PTFE and superglue. (1.4) Ethical implications—drugs, additives and pesticides can have harmful effects on both people and the environment. (4.5) Under ...
... Essential idea: Organic chemistry focuses on the chemistry of compounds containing carbon. Nature of science: Serendipity and scientific discoveries—PTFE and superglue. (1.4) Ethical implications—drugs, additives and pesticides can have harmful effects on both people and the environment. (4.5) Under ...
Chapter 3 Molecules, Compounds, and Chemical Equations
... 9The formulas for ionic compounds are empirical. The empirical formula for the ionic compound fluorspar is CaCl2. This means that there is 1 Ca2+ ion for every 2 Cl− ions in the compound. The empirical formula for the molecular compound oxalic acid is CHO2. This means that there is 1 C atom and 1 H ...
... 9The formulas for ionic compounds are empirical. The empirical formula for the ionic compound fluorspar is CaCl2. This means that there is 1 Ca2+ ion for every 2 Cl− ions in the compound. The empirical formula for the molecular compound oxalic acid is CHO2. This means that there is 1 C atom and 1 H ...
ch 1: organic chemistry
... can also be classified by the type of C where the –OH is attached since C can form 4 bonds, the C atom can be attached to 1, 2 or 3 alkyl groups resulting in 1°, 2° and 3° alcohols example 1-propanol is a primary alcohol, 2-propanol is a secondary alcohol, while 2-methyl-2-propanol is a tertiary ...
... can also be classified by the type of C where the –OH is attached since C can form 4 bonds, the C atom can be attached to 1, 2 or 3 alkyl groups resulting in 1°, 2° and 3° alcohols example 1-propanol is a primary alcohol, 2-propanol is a secondary alcohol, while 2-methyl-2-propanol is a tertiary ...
Lecture 14a - UCLA Chemistry and Biochemistry
... Solvent: DMSO (cannot be used in Chem 30CL), PTC conditions, solid state reaction An one-pot reaction is not advisable here because the reactants, the intermediate and the product are very difficult to separate from each other (anhydrous ZnI2 is not available!) The Corey-Chaykovsky reagent can ...
... Solvent: DMSO (cannot be used in Chem 30CL), PTC conditions, solid state reaction An one-pot reaction is not advisable here because the reactants, the intermediate and the product are very difficult to separate from each other (anhydrous ZnI2 is not available!) The Corey-Chaykovsky reagent can ...
Section 8.3 Names and Formulas of Ionic Compounds Formula Unit
... 1. Name the cation first and the anion second 2. Monoatomic cations use the element. 3. Monoatomic anions take their name from the root of the element name plus the suffix -ide. 4. Group 1A and 2A metals have only one oxidation number. Transition metals and metals on the right side of the periodic t ...
... 1. Name the cation first and the anion second 2. Monoatomic cations use the element. 3. Monoatomic anions take their name from the root of the element name plus the suffix -ide. 4. Group 1A and 2A metals have only one oxidation number. Transition metals and metals on the right side of the periodic t ...
Part II - American Chemical Society
... c. XeF2 is nonpolar. Both Xe–F bond dipoles are the same size, but due to the linear geometry they offset each other. XeF4 is nonpolar. All Xe–F bond dipoles are the same size, but due to the square planar geometry they offset each other. XeO3 is polar. The Xe–O bond dipoles are the same size, and t ...
... c. XeF2 is nonpolar. Both Xe–F bond dipoles are the same size, but due to the linear geometry they offset each other. XeF4 is nonpolar. All Xe–F bond dipoles are the same size, but due to the square planar geometry they offset each other. XeO3 is polar. The Xe–O bond dipoles are the same size, and t ...
Identification of Unknown Organic Compounds
... The identification and characterization of the structures of unknown substances are an important part of organic chemistry. Although it is often possible to establish the structure of a compound on the basis of spectra alone (IR, NMR, etc.), the spectra typically must be supplemented with other info ...
... The identification and characterization of the structures of unknown substances are an important part of organic chemistry. Although it is often possible to establish the structure of a compound on the basis of spectra alone (IR, NMR, etc.), the spectra typically must be supplemented with other info ...
Course Syllabus - San Diego Mesa College
... Learning Pub., 1999. Student Guide and Solution Manual by Susan McMurray. The books can be purchased at the Mesa bookstore. Course Description, Goals, and Objectives; This is the first semester of one year course in Organic Chemistry. It is designed primarily for science majors. Major themes include ...
... Learning Pub., 1999. Student Guide and Solution Manual by Susan McMurray. The books can be purchased at the Mesa bookstore. Course Description, Goals, and Objectives; This is the first semester of one year course in Organic Chemistry. It is designed primarily for science majors. Major themes include ...
1 - vnhsteachers
... 5A. CH3CH2CH3 6. An alkyne contains two carbon atoms. Provide its condensed structural formula. 6A. C2H2 7. Nitrogen added to alkanes forms: 7A. amines 8. Organic chemistry is the study of the __________ atom. 8A. carbon 9. The strongest chemical bonds belong to: 9A. carbon 10. Specific arrangement ...
... 5A. CH3CH2CH3 6. An alkyne contains two carbon atoms. Provide its condensed structural formula. 6A. C2H2 7. Nitrogen added to alkanes forms: 7A. amines 8. Organic chemistry is the study of the __________ atom. 8A. carbon 9. The strongest chemical bonds belong to: 9A. carbon 10. Specific arrangement ...
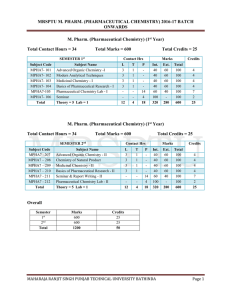
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
... Optical Isomerism in Compounds Containing No Chiral Atom: Biphenyls, Allenes, Compounds with Exocylic Double Bonds, Spiranes, Chirality due to a Helical Shape, Chirality caused by Restricted Rotation of other Types. Cis-Trans Isomerism: Resulting from Double Bonds, Monocyclic Compounds, Fused Ring S ...
Additional Structures to Accompany Exp
... Table 18A.3 Complete the table by creating the cycloalkane with the appropriate number of carbons. Number of C ...
... Table 18A.3 Complete the table by creating the cycloalkane with the appropriate number of carbons. Number of C ...
SORAN UNIVERSITY
... Students will have an understanding through study different experiments in organic laboratory, like recrystallizaion, functional groups tests , solubility tests for different organic families example carboxylic acid , amines , esters, alcohols and other different compounds, and the most important th ...
... Students will have an understanding through study different experiments in organic laboratory, like recrystallizaion, functional groups tests , solubility tests for different organic families example carboxylic acid , amines , esters, alcohols and other different compounds, and the most important th ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.