Chapter 17 Aldehydes and Ketones
... • As with any other equilibrium, we can drive it in either direction by using Le Chatelier's principle. • To drive it to the right, we either use a large excess of alcohol or remove water from the equilibrium mixture ...
... • As with any other equilibrium, we can drive it in either direction by using Le Chatelier's principle. • To drive it to the right, we either use a large excess of alcohol or remove water from the equilibrium mixture ...
Wed March 3 lecture
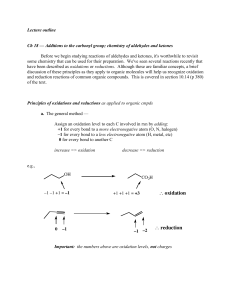
... Ch 18 — Additions to the carbonyl group; chemistry of aldehydes and ketones Before we begin studying reactions of aldehydes and ketones, it's worthwhile to revisit some chemistry that can be used for their preparation. We've seen several reactions recently that have been described as oxidations or r ...
... Ch 18 — Additions to the carbonyl group; chemistry of aldehydes and ketones Before we begin studying reactions of aldehydes and ketones, it's worthwhile to revisit some chemistry that can be used for their preparation. We've seen several reactions recently that have been described as oxidations or r ...
Investigation 8
... It is interesting that the experimental value of ethanol -while still on the line- appears as lower than the other experimental values. This is not observed in the values based on BE where the five energy values are perfectly aligned. Ethanol´s value is clearly lower than that of methanol and slight ...
... It is interesting that the experimental value of ethanol -while still on the line- appears as lower than the other experimental values. This is not observed in the values based on BE where the five energy values are perfectly aligned. Ethanol´s value is clearly lower than that of methanol and slight ...
Revision Y12 Chemistry PLC
... linear, non-linear, trigonal planar, pyramidal, tetrahedral and octahedral. Lone pairs repel more than bonded pairs and the bond angles for common examples of each shape including CH4 (109.5°), NH3 (107°) and H2O (104.5°). Electronegativity and bond polarity (i) electronegativity as the ability of a ...
... linear, non-linear, trigonal planar, pyramidal, tetrahedral and octahedral. Lone pairs repel more than bonded pairs and the bond angles for common examples of each shape including CH4 (109.5°), NH3 (107°) and H2O (104.5°). Electronegativity and bond polarity (i) electronegativity as the ability of a ...
Organic Nomenclature
... Organic compounds are compounds containing carbon bonded to other nonmetals such as hydrogen, nitrogen, oxygen, or the halogens. The term organic comes from the old idea that all carbon containing compounds had to be produced by a living organism. While living organisms produce a vast number of orga ...
... Organic compounds are compounds containing carbon bonded to other nonmetals such as hydrogen, nitrogen, oxygen, or the halogens. The term organic comes from the old idea that all carbon containing compounds had to be produced by a living organism. While living organisms produce a vast number of orga ...
Abbreviated Chapter 17 Powerpoint
... • Anisole undergoes nitration about 10,000 times faster than benzene and about 400 times faster than toluene. • This result seems curious because oxygen is a strongly electronegative group, yet it donates electron density to stabilize the transition state and the sigma complex. ...
... • Anisole undergoes nitration about 10,000 times faster than benzene and about 400 times faster than toluene. • This result seems curious because oxygen is a strongly electronegative group, yet it donates electron density to stabilize the transition state and the sigma complex. ...
Alcohols
... carbon atom it is primary; two other carbon atoms – secondary and three carbon atoms – tertiary. Properties of Alcohols The hydroxyl group in an alcohol is polar therefore hydrogen bonding occurs. “Like dissolves Like” therefore these molecules are soluble in other polar solvents. However, in lo ...
... carbon atom it is primary; two other carbon atoms – secondary and three carbon atoms – tertiary. Properties of Alcohols The hydroxyl group in an alcohol is polar therefore hydrogen bonding occurs. “Like dissolves Like” therefore these molecules are soluble in other polar solvents. However, in lo ...
The aim of the work
... is directed to the atom H1 along the C(2)-H1 bond. With the approach of the hydroxyl group to a distance of 2.86 Å the water molecule removes. The elimination of H1 from C(2) atom by OH- ion results in leaving of the second water molecule and unsaturated compound 42 formation. The interaction of pro ...
... is directed to the atom H1 along the C(2)-H1 bond. With the approach of the hydroxyl group to a distance of 2.86 Å the water molecule removes. The elimination of H1 from C(2) atom by OH- ion results in leaving of the second water molecule and unsaturated compound 42 formation. The interaction of pro ...
Chapter 14 – Organic Chemistry
... - study of carbon and carbon compounds Organic Compounds: - compounds which contain both carbon and hydrogen - carbon atoms bond together to form chains, branches, rings, or networks Characteristics of Organic Compounds: - covalent bonding - carbon forms 4 bonds to make a tetrahedron – results in LA ...
... - study of carbon and carbon compounds Organic Compounds: - compounds which contain both carbon and hydrogen - carbon atoms bond together to form chains, branches, rings, or networks Characteristics of Organic Compounds: - covalent bonding - carbon forms 4 bonds to make a tetrahedron – results in LA ...
sOLUBILITY
... between polar covalent molecules that possess a hydrogen bonded to an extremely electronegative element, specifically - N, O, and F ...
... between polar covalent molecules that possess a hydrogen bonded to an extremely electronegative element, specifically - N, O, and F ...
Chapter 7-8-9
... a. only when melted b. only when dissolved c. only when it is in crystal form d. only when melted or dissolved in water Covalent compounds display which of these properties? a. They are hard, brittle solids b. They have high melting and boiling points c. They display luster. d. Their intermolecular ...
... a. only when melted b. only when dissolved c. only when it is in crystal form d. only when melted or dissolved in water Covalent compounds display which of these properties? a. They are hard, brittle solids b. They have high melting and boiling points c. They display luster. d. Their intermolecular ...
sample paper - CBSE PORTAL
... Q.2 People living at high altitude generally suffer from anoxia , Explain. Q.3 Define limiting molar conductivity . Q.4 “A greater insight in to the energetic and mechanistic aspects of reactions”. Explain the above statement which was given by “Max Trautz” and William Lewis” Q.5 Name the sweetest c ...
... Q.2 People living at high altitude generally suffer from anoxia , Explain. Q.3 Define limiting molar conductivity . Q.4 “A greater insight in to the energetic and mechanistic aspects of reactions”. Explain the above statement which was given by “Max Trautz” and William Lewis” Q.5 Name the sweetest c ...
ch12 by dina
... Organolithium and Grignard reagents behave as if they were carbanions and they are therefore very strong bases They react readily with hydrogen atoms attached to oxygen, nitrogen ...
... Organolithium and Grignard reagents behave as if they were carbanions and they are therefore very strong bases They react readily with hydrogen atoms attached to oxygen, nitrogen ...
File - Pedersen Science
... explains its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isomers: structural, geometric, and enantiomer ...
... explains its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isomers: structural, geometric, and enantiomer ...
Chapter 4
... its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isomers: structural, geometric, and enantiomer ...
... its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isomers: structural, geometric, and enantiomer ...
Grant MacEwan College - Faculty Web Pages
... Course Description:(3 credits) This course studies the molecular structure and reactivity of organic compounds based on their functional groups and is intended for students who have obtained at least three credits in Introductory University Chemistry. The course provides an introduction to nomenclat ...
... Course Description:(3 credits) This course studies the molecular structure and reactivity of organic compounds based on their functional groups and is intended for students who have obtained at least three credits in Introductory University Chemistry. The course provides an introduction to nomenclat ...
Chapter 4
... explains its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isomers: structural, geometric, and enantiomer ...
... explains its ability to form large, complex, diverse organic molecules 2. Describe how carbon skeletons may vary and explain how this variation contributes to the diversity and complexity of organic molecules 3. Distinguish among the three types of isomers: structural, geometric, and enantiomer ...
Chapter 14
... Chapter 14 - Organic Compounds containing Oxygen, Halogen or Sulfur Alcohols, Ethers, Alkyl halides & Thiols ROH RX ROR RSH All of these compounds contain a carbon atom that is singly bonded to a heteroatom! Alcohols & ethers are organic derivatives of water - Replacing H with one or two alkyl group ...
... Chapter 14 - Organic Compounds containing Oxygen, Halogen or Sulfur Alcohols, Ethers, Alkyl halides & Thiols ROH RX ROR RSH All of these compounds contain a carbon atom that is singly bonded to a heteroatom! Alcohols & ethers are organic derivatives of water - Replacing H with one or two alkyl group ...
avogadro exam 2001 - University of Waterloo
... 12 A U-tube mercury manometer is open on the right arm and connected to a gas sample at the other arm as shown below. The atmospheric pressure is 101 kPa and the difference in levels of mercury is 20 cm. What is the pressure of the gas in the bulb? ...
... 12 A U-tube mercury manometer is open on the right arm and connected to a gas sample at the other arm as shown below. The atmospheric pressure is 101 kPa and the difference in levels of mercury is 20 cm. What is the pressure of the gas in the bulb? ...
5 Alkenes and Alkynes GOB Structures
... • are named by assigning the double bond to be between carbon 1 and carbon 2 when a substituent is on the ring. • do not need to include the numbers for the double bond. 3-methylcyclopentene (It is understood that the double bond is between carbon 1 and carbon 2.) ...
... • are named by assigning the double bond to be between carbon 1 and carbon 2 when a substituent is on the ring. • do not need to include the numbers for the double bond. 3-methylcyclopentene (It is understood that the double bond is between carbon 1 and carbon 2.) ...
Classes of organic acids and bases
... p orbitals w/ total of 4 e-. They form a 3-atom π molecular orbital system; ½ π connects C to each O. ...
... p orbitals w/ total of 4 e-. They form a 3-atom π molecular orbital system; ½ π connects C to each O. ...
Aldehid dan Keton
... Boiling Points • More polar, so higher boiling point than comparable alkane or ether. • Cannot H-bond to each other, so lower boiling point than comparable alcohol. ...
... Boiling Points • More polar, so higher boiling point than comparable alkane or ether. • Cannot H-bond to each other, so lower boiling point than comparable alcohol. ...
Writing Chemical Formulas and Chemical Reactions
... All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired number (coefficient ...
... All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired number (coefficient ...
BioN02 Introduction to organic chemistry Summer 2014
... The hydroxyl group takes precedence over alkyl groups and halogen substituents, as well as double bonds, in the numbering of the parent chain Alcohols may also be classified as primary, 1º, secondary, 2º & tertiary, 3º (carbons attached to the carbon with –OH) ...
... The hydroxyl group takes precedence over alkyl groups and halogen substituents, as well as double bonds, in the numbering of the parent chain Alcohols may also be classified as primary, 1º, secondary, 2º & tertiary, 3º (carbons attached to the carbon with –OH) ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.