Adv_H_Unit_3_Pupil_N.. - Chemistry Teaching Resources
... Heterolytic fission is more likely when a bond is already polar. For example, bromomethane contains a polar carbon to bromine bond and under certain conditions this can break heterolytically (Figure 1). Figure 1 ...
... Heterolytic fission is more likely when a bond is already polar. For example, bromomethane contains a polar carbon to bromine bond and under certain conditions this can break heterolytically (Figure 1). Figure 1 ...
CHEM 121. Chapter 15
... (2) The linear notation for an aldehyde is RCOH and that for a ketone is RCOR. (3) Water molecules can hydrogen bond with aldehyde and ketone molecules. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true ...
... (2) The linear notation for an aldehyde is RCOH and that for a ketone is RCOR. (3) Water molecules can hydrogen bond with aldehyde and ketone molecules. A) All three statements are true. B) Two of the three statements are true. C) Only one of the statements is true. D) None of the statements is true ...
Alcohols and Thiols
... base in many of the reaction you’ve seen. Alcohols can be deprotonated by many of the strong bases you’ve seen, such as sodium hydride, sodamide, nearly all alkyl-metal compounds (e.g. methyl lithium, Grignard reagents, sodium acetylide). These are all good methods for the preparation of alkoxides ( ...
... base in many of the reaction you’ve seen. Alcohols can be deprotonated by many of the strong bases you’ve seen, such as sodium hydride, sodamide, nearly all alkyl-metal compounds (e.g. methyl lithium, Grignard reagents, sodium acetylide). These are all good methods for the preparation of alkoxides ( ...
ii. year course contents
... interactions, boiling, melting, dissolution, molecular and structural formulas, isomer) Introduction to Organic Chemistry - The Basic Concepts (acidity and alkalinity, organic reactions, energy diagrams, some general definitions). Functional groups and nomenclature (Alkanes and the general naming ru ...
... interactions, boiling, melting, dissolution, molecular and structural formulas, isomer) Introduction to Organic Chemistry - The Basic Concepts (acidity and alkalinity, organic reactions, energy diagrams, some general definitions). Functional groups and nomenclature (Alkanes and the general naming ru ...
Chapters E-18 review - Bakersfield College
... 10. How can we convert thé first compound into thé second compound? Détermine thé type of chemical reaction, catalyst (if it is necessary) and other necessary conditions: a) Benzène to Chlorobenzene ...
... 10. How can we convert thé first compound into thé second compound? Détermine thé type of chemical reaction, catalyst (if it is necessary) and other necessary conditions: a) Benzène to Chlorobenzene ...
Organic Chemistry Fifth Edition
... Summary of reactions of ethers No reactions of ethers encountered to this point. Ethers are relatively unreactive. Their low level of reactivity is one reason why ethers are often used as solvents in chemical reactions, and as protecting groups for reactive –OH group. Ethers oxidize in air to form ...
... Summary of reactions of ethers No reactions of ethers encountered to this point. Ethers are relatively unreactive. Their low level of reactivity is one reason why ethers are often used as solvents in chemical reactions, and as protecting groups for reactive –OH group. Ethers oxidize in air to form ...
2 - AQA
... to use helium as its lifting gas, rather than hydrogen, but the only source of large volumes of helium was the USA and they refused to sell it to Germany because of Hitler’s aggressive policies. The airship was therefore made to use hydrogen. It held about 210 000 m3 of hydrogen gas but this volume ...
... to use helium as its lifting gas, rather than hydrogen, but the only source of large volumes of helium was the USA and they refused to sell it to Germany because of Hitler’s aggressive policies. The airship was therefore made to use hydrogen. It held about 210 000 m3 of hydrogen gas but this volume ...
apch04 test review_ans
... blue dye in a solution. Our calorimeters have three options for wavelengths: Red - 620 nm, Green - 550 nm, Blue - 470 nm. Based on the absorption spectrum for the three dyes in the figure below, which settings is the best setting for a lab measuring the concentration of each? Absorbance measurements ...
... blue dye in a solution. Our calorimeters have three options for wavelengths: Red - 620 nm, Green - 550 nm, Blue - 470 nm. Based on the absorption spectrum for the three dyes in the figure below, which settings is the best setting for a lab measuring the concentration of each? Absorbance measurements ...
Chemistry
... Chapter 18 (electrochemistry) is not included on this Review Guide but will be on the Exam. Use the notes, handouts, homework, and textbook to review. It’s a short chapter and the topic we covered most recently, so it should be fresh in your mind. Chapter 8 – The Mole and Chemical Composition Topics ...
... Chapter 18 (electrochemistry) is not included on this Review Guide but will be on the Exam. Use the notes, handouts, homework, and textbook to review. It’s a short chapter and the topic we covered most recently, so it should be fresh in your mind. Chapter 8 – The Mole and Chemical Composition Topics ...
Chem101, 2nd Major Exam, term061
... 12. Which one of the following statements is TRUE? The electron affinity of bromine (Br) is greater than that of selenium (Se). The first ionization energy of hydrogen (H) is greater than that of helium (He). The first ionization energy of phosphorus (P) is less than that of sulfur (S). The fourth i ...
... 12. Which one of the following statements is TRUE? The electron affinity of bromine (Br) is greater than that of selenium (Se). The first ionization energy of hydrogen (H) is greater than that of helium (He). The first ionization energy of phosphorus (P) is less than that of sulfur (S). The fourth i ...
Chapter -
... elements.(never change subscripts!) 2. Treat polyatomic ions as units rather than individual elements. 3. Count carefully, being sure to recount after each coefficient change. ...
... elements.(never change subscripts!) 2. Treat polyatomic ions as units rather than individual elements. 3. Count carefully, being sure to recount after each coefficient change. ...
DEPARTMENT OF CHEMISTRY Course Book for M.Sc. in Chemistry
... Transition metal Chemistry: Introduction: Structure of metal ligand complexes, coordination no., isomerism, thermodynamic stability, stability constant, Irving –Willium series, Chelate and macrocyclic effect Theories of bonding : VBT, CFT, d-orbital splitting in octahedral, JT-distorted octahedral, ...
... Transition metal Chemistry: Introduction: Structure of metal ligand complexes, coordination no., isomerism, thermodynamic stability, stability constant, Irving –Willium series, Chelate and macrocyclic effect Theories of bonding : VBT, CFT, d-orbital splitting in octahedral, JT-distorted octahedral, ...
Collins CSEC® Chemistry Workbook answers A1 States of matter
... 3. a) The copper atoms are packed together in rows and the valence electrons from each atom become delocalised. This forms positive copper cations and a sea of mobile electrons. The strong electrostatic forces of attraction between the delocalised electrons and the cations, called the metallic bond, ...
... 3. a) The copper atoms are packed together in rows and the valence electrons from each atom become delocalised. This forms positive copper cations and a sea of mobile electrons. The strong electrostatic forces of attraction between the delocalised electrons and the cations, called the metallic bond, ...
Aldehydes, Ketones and Carboxylic Acids
... compounds with functional groups containing carbonoxygen single bond. In this Unit, we will study about the organic compounds containing carbon-oxygen double bond (>C=O) called carbonyl group, which is one of the most important functional groups in organic chemistry. In aldehydes, the carbonyl group ...
... compounds with functional groups containing carbonoxygen single bond. In this Unit, we will study about the organic compounds containing carbon-oxygen double bond (>C=O) called carbonyl group, which is one of the most important functional groups in organic chemistry. In aldehydes, the carbonyl group ...
Course Book - Department of Chemistry
... Students will gain the fundamental knowledge about the synthesis, structure, bonding and properties of some selected main group elements. Exposure to the fundamental concepts on different theories of bonding and their relation to the properties of transition metal coordination compounds will be help ...
... Students will gain the fundamental knowledge about the synthesis, structure, bonding and properties of some selected main group elements. Exposure to the fundamental concepts on different theories of bonding and their relation to the properties of transition metal coordination compounds will be help ...
unit 6 alcohols
... LAH Reduction of Carbonyls When using LiAlH4, H2O may not be included with it but must be added afterward (in a second reaction step). Because Al is less electronegative than B, LiAlH4 is a stronger nucleophile and will react with less reactive carbonyls such as esters, as well as with the more ...
... LAH Reduction of Carbonyls When using LiAlH4, H2O may not be included with it but must be added afterward (in a second reaction step). Because Al is less electronegative than B, LiAlH4 is a stronger nucleophile and will react with less reactive carbonyls such as esters, as well as with the more ...
Acrobat () verson
... tall. Suppose a column of Hg is set up where the bath is open to the atmosphere, and the column height of Hg is 760.0 mm with the top of the enclosed column being a vacuum. Next, suppose some diethyl ether (a volatile liquid) is injected into the top of the vacuum above the Hg column such that the s ...
... tall. Suppose a column of Hg is set up where the bath is open to the atmosphere, and the column height of Hg is 760.0 mm with the top of the enclosed column being a vacuum. Next, suppose some diethyl ether (a volatile liquid) is injected into the top of the vacuum above the Hg column such that the s ...
12 - einstein classes
... by a lone pair, and the bond angle F — N — F is 102030’. However, the dipole moment is very low (0.23 Debye units) compared with 1.47D for NH3. The highly electronegative F atoms attract electrons, and these moments partly cancel the moment from the lone pair, and this reduces both the dipole moment ...
... by a lone pair, and the bond angle F — N — F is 102030’. However, the dipole moment is very low (0.23 Debye units) compared with 1.47D for NH3. The highly electronegative F atoms attract electrons, and these moments partly cancel the moment from the lone pair, and this reduces both the dipole moment ...
Chapter 22 - 2012 Book Archive
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
Practice Problem - HCC Southeast Commons
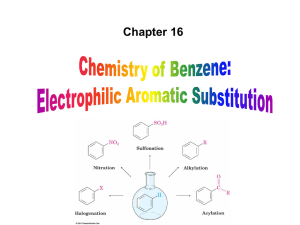
... intermediate loses H+ to regenerate the aromatic ring and yield a substitution product in which H+ is replaced by Br+ – It is similar to the 2nd step of an E1 reaction – The carbocation intermediate transfers a H+ to FeBr4- (from Br- and FeBr3) – This restores aromaticity (in contrast with addition ...
... intermediate loses H+ to regenerate the aromatic ring and yield a substitution product in which H+ is replaced by Br+ – It is similar to the 2nd step of an E1 reaction – The carbocation intermediate transfers a H+ to FeBr4- (from Br- and FeBr3) – This restores aromaticity (in contrast with addition ...
Chapter 8
... Amines are classified as 1°, 2°, or 3° depending on the number of carbon groups bonded to nitrogen. ...
... Amines are classified as 1°, 2°, or 3° depending on the number of carbon groups bonded to nitrogen. ...
Synthesis of New 3-Heteroarylindoles as Potential
... MCF‐7 human breast carcinoma cell line. thiazole and 1,3,4‐thiadiazole have antitumor activity with excellent IG50 and IC50 as depicted in activity with excellent IG50 and IC50 as depicted in Figure 1 [33–37]. In view of these facts, we report Figure 1 [33–37]. In view of these facts, ...
... MCF‐7 human breast carcinoma cell line. thiazole and 1,3,4‐thiadiazole have antitumor activity with excellent IG50 and IC50 as depicted in activity with excellent IG50 and IC50 as depicted in Figure 1 [33–37]. In view of these facts, we report Figure 1 [33–37]. In view of these facts, ...
M for Moles - Shop
... molecular weights and their relationships to moles and chemical equations? This e-book teaches you how to do mole calculations that are often encountered in chemistry. Readers are assumed to have only a basic science concept and with minimum knowledge in chemistry. This e-book will show you how to s ...
... molecular weights and their relationships to moles and chemical equations? This e-book teaches you how to do mole calculations that are often encountered in chemistry. Readers are assumed to have only a basic science concept and with minimum knowledge in chemistry. This e-book will show you how to s ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.