- Angelo State University
... The Molar Mass of a Compound • The molar mass of a compound is obtained by adding together the atomic masses of all of the atoms in the molecule or formula unit. This number is either the mass of the compound in units of amu, or the mass of one mole of the compound in grams. – For molecular compound ...
... The Molar Mass of a Compound • The molar mass of a compound is obtained by adding together the atomic masses of all of the atoms in the molecule or formula unit. This number is either the mass of the compound in units of amu, or the mass of one mole of the compound in grams. – For molecular compound ...
IR handout
... or acyl halide. The an aldehyde may be confirmed with C-H absorption from 2840 to 2720 cm-1. 3. the O-H or N-H absorption between 3200 and 3600 cm-1. This indicates either an alcohol, N-H containing amine or amide, or carboxylic acid. For -NH2 a doublet will be observed. 4.the C-O absorption between ...
... or acyl halide. The an aldehyde may be confirmed with C-H absorption from 2840 to 2720 cm-1. 3. the O-H or N-H absorption between 3200 and 3600 cm-1. This indicates either an alcohol, N-H containing amine or amide, or carboxylic acid. For -NH2 a doublet will be observed. 4.the C-O absorption between ...
CHAPTER 17: Carbonyl group (1)
... presence of H2O and acid exists in the form of a hydrate. The hydrate is then further oxidized to the carboxylic acid. Secondary alcohols produce ketones, while tertiary alcohols remain intact. ...
... presence of H2O and acid exists in the form of a hydrate. The hydrate is then further oxidized to the carboxylic acid. Secondary alcohols produce ketones, while tertiary alcohols remain intact. ...
Chemistry - Andhra University
... Identification of alcohols by oxidation with KMnO4, ceric ammonium nitrate, lucas reagent and phenols by reaction with FeCl3. Polyhydroxy compounds: Pinacol-Pinacolone rearrangement. 3. Carbonyl compounds 10 h Nomenclature of aliphatic and aromatic carbonyl compounds, structure of the carbonyl group ...
... Identification of alcohols by oxidation with KMnO4, ceric ammonium nitrate, lucas reagent and phenols by reaction with FeCl3. Polyhydroxy compounds: Pinacol-Pinacolone rearrangement. 3. Carbonyl compounds 10 h Nomenclature of aliphatic and aromatic carbonyl compounds, structure of the carbonyl group ...
Keq = [A] [B] [C] [D]
... b) Substitute the concentration terms with the equilibrium values from the ICE table, and the value of Kc: 6.0 x 10-9 = x · x c) Solve for x by taking the square root of both sides: 6.0 x 10-9 = x2 and x = 7.7 x 10-5 mol/L d) Therefore, the concentration of both NH3 and HCl gases at equilibrium wil ...
... b) Substitute the concentration terms with the equilibrium values from the ICE table, and the value of Kc: 6.0 x 10-9 = x · x c) Solve for x by taking the square root of both sides: 6.0 x 10-9 = x2 and x = 7.7 x 10-5 mol/L d) Therefore, the concentration of both NH3 and HCl gases at equilibrium wil ...
naming of ethers
... Solubility in water – low molecular mass ethers are soluble in water. As the mass of a molecule (size of the alkyl group) increases, the solubility decreases. Melting and Boiling points – the melting and boiling points of ethers are lower than isomeric alcohols. Flammability – like alcohols, ethers ...
... Solubility in water – low molecular mass ethers are soluble in water. As the mass of a molecule (size of the alkyl group) increases, the solubility decreases. Melting and Boiling points – the melting and boiling points of ethers are lower than isomeric alcohols. Flammability – like alcohols, ethers ...
Li K-edge XANES and Li(1s) XPS Spectra of Lithium Compounds
... Li K-edge XANES and Li(1s) XPS spectra of various lithium complexes can be measured under the suitable measuring conditions. A great variety of spectra have been obtained. Li K-edge XANES spectra are shown in Fig. 2. They have four or five peaks with a variety of peak intensities. The shapes and pos ...
... Li K-edge XANES and Li(1s) XPS spectra of various lithium complexes can be measured under the suitable measuring conditions. A great variety of spectra have been obtained. Li K-edge XANES spectra are shown in Fig. 2. They have four or five peaks with a variety of peak intensities. The shapes and pos ...
Alcohols - Chem1-tsu
... An alcohol contains one or more hydroxyl (OH) groups(s) attached to aliphatic carbon atom(s). For e.g., ethyl alcohol formula CH3, CH2, OH is an alcohol. A phenol contains -OH group(s) directly attached to an aryl carbon atom(s). The simplest phenol is hydroxybenzene also called phenol with formula ...
... An alcohol contains one or more hydroxyl (OH) groups(s) attached to aliphatic carbon atom(s). For e.g., ethyl alcohol formula CH3, CH2, OH is an alcohol. A phenol contains -OH group(s) directly attached to an aryl carbon atom(s). The simplest phenol is hydroxybenzene also called phenol with formula ...
Chapter 16_CHEM 131
... • The N-H bond is not quite as polar as the O-H bond. • Primary and secondary amines can form hydrogen bonds between molecules. • The hydrogen bonds are not as strong as those of alcohols, so amine boiling points are somewhat lower than those of alcohols. • Simple, low molecular weight amines are ga ...
... • The N-H bond is not quite as polar as the O-H bond. • Primary and secondary amines can form hydrogen bonds between molecules. • The hydrogen bonds are not as strong as those of alcohols, so amine boiling points are somewhat lower than those of alcohols. • Simple, low molecular weight amines are ga ...
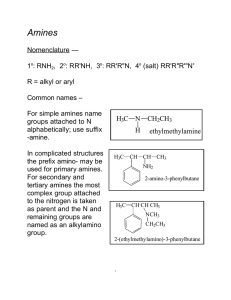
Chapter 21: Amines. Organic derivatives of ammonia, NH3. Nitrogen
... Anilines are so activated that multiple substitution reactions can be a problem. The reactivity of the acetamide is attenuated so that mono-substitution is achieved. The acetamide group is compatable with Friedel-Crafts reactions. ...
... Anilines are so activated that multiple substitution reactions can be a problem. The reactivity of the acetamide is attenuated so that mono-substitution is achieved. The acetamide group is compatable with Friedel-Crafts reactions. ...
5.1 questions - DrBravoChemistry
... Calculate the standard enthalpy change and the standard entropy change for this reaction. Standard enthalpy change ........................................................................... ...
... Calculate the standard enthalpy change and the standard entropy change for this reaction. Standard enthalpy change ........................................................................... ...
Determination of Cystein and Methionine by Oscillating Chemical
... new chemical oscillators. A successful mechanistic study also contributes to the knowledge of the general chemistry and kinetics of the chemical species that are involved. In this study conductometry method was used to characterize the oscillating conductance in the system H2O2–KSCN–CuSO4–NaOH. The ...
... new chemical oscillators. A successful mechanistic study also contributes to the knowledge of the general chemistry and kinetics of the chemical species that are involved. In this study conductometry method was used to characterize the oscillating conductance in the system H2O2–KSCN–CuSO4–NaOH. The ...
ch13[1].
... • A new carbon-carbon bond is formed in the process. • We study addition of carbon nucleophiles called Grignard reagents. • Victor Grignard was awarded the Nobel Prize for chemistry in 1912 for their discovery and application to organic synthesis. • Grignard reagents have the general formula RMgX, w ...
... • A new carbon-carbon bond is formed in the process. • We study addition of carbon nucleophiles called Grignard reagents. • Victor Grignard was awarded the Nobel Prize for chemistry in 1912 for their discovery and application to organic synthesis. • Grignard reagents have the general formula RMgX, w ...
NOBLE-GAS CHEMISTRY
... adducts of noble gases to a metal center, NgBe(II)O, where Ng = Ar, Kr, and Xe.31 The case of XeBeO is rather straightforward: a coordinatively unsaturated Be(II) cation exposes its empty (sp) hybrid, and is ready to bind whatever Lewis base you provide. Hence, it will bind a Xe atom with a surprisi ...
... adducts of noble gases to a metal center, NgBe(II)O, where Ng = Ar, Kr, and Xe.31 The case of XeBeO is rather straightforward: a coordinatively unsaturated Be(II) cation exposes its empty (sp) hybrid, and is ready to bind whatever Lewis base you provide. Hence, it will bind a Xe atom with a surprisi ...
Unit 4/5 packet
... The following ionic compounds contain polyatomic ions (ions like NO31-, SO42- or OH1- which are made up of several atoms bonded together). Whenever a polyatomic ion needs to be doubled or tripled in a formula, parentheses must be used to avoid confusion. For example: magnesium nitrate = Mg(NO3)2 [no ...
... The following ionic compounds contain polyatomic ions (ions like NO31-, SO42- or OH1- which are made up of several atoms bonded together). Whenever a polyatomic ion needs to be doubled or tripled in a formula, parentheses must be used to avoid confusion. For example: magnesium nitrate = Mg(NO3)2 [no ...
Alcohols
... HYDROXIDE ION (OH-) WHICH IS A NEGATIVELY CHARGED PARTICLE FOUND IN BASES. IN BASES, THE HYDROXIDE ION IS HELD TO A POSITIVE ION BY AN A IONIC BOND. • ALCOHOLS HAVE THE GENERAL FORMULA CnH 2n+ 1OH ...
... HYDROXIDE ION (OH-) WHICH IS A NEGATIVELY CHARGED PARTICLE FOUND IN BASES. IN BASES, THE HYDROXIDE ION IS HELD TO A POSITIVE ION BY AN A IONIC BOND. • ALCOHOLS HAVE THE GENERAL FORMULA CnH 2n+ 1OH ...
NH 4 1+
... These rules would enable you to predict, for example, that Mg(NO3)2 is soluble – since all NO31- compounds are soluble – no exceptions. On the other hand, CaS would be insoluble, since S2- compounds are insoluble. (There are exceptions, but Ca is not among them.) Most Br1- compounds are soluble, but ...
... These rules would enable you to predict, for example, that Mg(NO3)2 is soluble – since all NO31- compounds are soluble – no exceptions. On the other hand, CaS would be insoluble, since S2- compounds are insoluble. (There are exceptions, but Ca is not among them.) Most Br1- compounds are soluble, but ...
1. True
... negative, then the quantity T ΔS is also negative, and subtracting a negative ΔS is actually adding it. So for ΔG to be negative, the positive T ΔS must be a smaller number than the negative ΔH. T ΔS will be smaller at low temperatures. 29. The standard free energy of formation of CS2(ℓ) is 65.3 kJ· ...
... negative, then the quantity T ΔS is also negative, and subtracting a negative ΔS is actually adding it. So for ΔG to be negative, the positive T ΔS must be a smaller number than the negative ΔH. T ΔS will be smaller at low temperatures. 29. The standard free energy of formation of CS2(ℓ) is 65.3 kJ· ...
Chapter 8 and 9
... From this information you can calculate the amount of carbon and hydrogen in the sample. However since oxygen is in excess you must find oxygen through indirect means (the mass comes from what is not accounted for by carbon and hydrogen, in a sample that only contains CHO). ...
... From this information you can calculate the amount of carbon and hydrogen in the sample. However since oxygen is in excess you must find oxygen through indirect means (the mass comes from what is not accounted for by carbon and hydrogen, in a sample that only contains CHO). ...
CHE 110 Dr. Nicholas Bizier Office DS 337b email
... From this information you can calculate the amount of carbon and hydrogen in the sample. However since oxygen is in excess you must find oxygen through indirect means (the mass comes from what is not accounted for by carbon and hydrogen, in a sample that only contains CHO). ...
... From this information you can calculate the amount of carbon and hydrogen in the sample. However since oxygen is in excess you must find oxygen through indirect means (the mass comes from what is not accounted for by carbon and hydrogen, in a sample that only contains CHO). ...
226 amines lec
... group eg -OH or -NR2. Azo compounds are often used as dyes. They are colored owing to extensive conjugation. For example, butter yellow was at one time used to color margarine yellow. It has been found to be carcinogenic and is no longer used for this purpose. ...
... group eg -OH or -NR2. Azo compounds are often used as dyes. They are colored owing to extensive conjugation. For example, butter yellow was at one time used to color margarine yellow. It has been found to be carcinogenic and is no longer used for this purpose. ...
IB Chemistry HL Topic5 Questions 1. Which
... Calculate the standard free energy change at 298 K, ΔGӨ, for the reaction in part (a). Use your answer and relevant information from part (d). If you did not obtain an answer to part (a), use ΔS Ө = –360 J K–1 (this is not the correct value). ...
... Calculate the standard free energy change at 298 K, ΔGӨ, for the reaction in part (a). Use your answer and relevant information from part (d). If you did not obtain an answer to part (a), use ΔS Ө = –360 J K–1 (this is not the correct value). ...
Chapter 16
... Therefore an aldehyde or ketone can be determined by comparing the melting points ...
... Therefore an aldehyde or ketone can be determined by comparing the melting points ...
Reductive Deoxygenation of Ketones and Secondary Alcohols by
... dialkyl ketones, as exemplified by the reduction of benzophenone, acetophenone and 5-nonanone, respectively. The corresponding secondary alcohols of these ketones, namely benzhydrol, l-phenyl-l-ethanol, and 5-nonanol, could also be converted into their respective methylene hydrocarbons by Lewis-acid ...
... dialkyl ketones, as exemplified by the reduction of benzophenone, acetophenone and 5-nonanone, respectively. The corresponding secondary alcohols of these ketones, namely benzhydrol, l-phenyl-l-ethanol, and 5-nonanol, could also be converted into their respective methylene hydrocarbons by Lewis-acid ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.



![Keq = [A] [B] [C] [D]](http://s1.studyres.com/store/data/014463360_1-50a2de0db1e8b9a361c4b31c6e85c28d-300x300.png)







![ch13[1].](http://s1.studyres.com/store/data/008194698_1-d188c504eac7b7806e762a2340484910-300x300.png)