Organic Halides (Haloalkanes) (Alkyl Halides)
... reaction with PBr3 which transforms OH- into a better leaving group allowing substitution (SN2) to occur without rearrangement. ...
... reaction with PBr3 which transforms OH- into a better leaving group allowing substitution (SN2) to occur without rearrangement. ...
fast pyrolysis characteristics of sugarcane bagasse hemicellulose
... easy to remove from the main chain and to degrade by the released volatiles.19 Composition of gas products The gas evolving profiles resulted from hemicellulose pyrolysis in the tubular furnace are plotted in Figure 3. The gas products released from the pyrolysis of hemicellulose were composed of CO ...
... easy to remove from the main chain and to degrade by the released volatiles.19 Composition of gas products The gas evolving profiles resulted from hemicellulose pyrolysis in the tubular furnace are plotted in Figure 3. The gas products released from the pyrolysis of hemicellulose were composed of CO ...
Chemistry
... aspects of general chemistry. Chemistry is mastered when students make the right connections in three key areas: topics that are related, conceptual reasoning with quantitative work, and the different modes of communicating information. McMurry/Fay’s Chemistry, Sixth Edition breaks through the tradi ...
... aspects of general chemistry. Chemistry is mastered when students make the right connections in three key areas: topics that are related, conceptual reasoning with quantitative work, and the different modes of communicating information. McMurry/Fay’s Chemistry, Sixth Edition breaks through the tradi ...
STOICHIOMETRY via ChemLog - Small
... use the exact amount of each reactant needed. Or, we may use an excess of some reactants and a limited amount of others. We may do this if one reactant is very expensive and others are inexpensive so that we can use all of the expensive compound. It can be more cost effective, even if we are wasting ...
... use the exact amount of each reactant needed. Or, we may use an excess of some reactants and a limited amount of others. We may do this if one reactant is very expensive and others are inexpensive so that we can use all of the expensive compound. It can be more cost effective, even if we are wasting ...
Oxidation catalytic system and oxidation process using the same
... N-hydroxyphthalimide), and a co-catalyst (except phospho vanadomolybdic acid) containing an element selected from the group consisting of Group 2A elements of the Periodic Table of Elements, transition metals (Group 3A to 7A elements, Group 8 elements, Group 1B elements and Group 2B elements of the ...
... N-hydroxyphthalimide), and a co-catalyst (except phospho vanadomolybdic acid) containing an element selected from the group consisting of Group 2A elements of the Periodic Table of Elements, transition metals (Group 3A to 7A elements, Group 8 elements, Group 1B elements and Group 2B elements of the ...
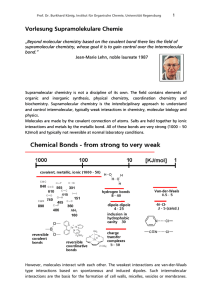
Vorlesung Supramolekulare Chemie
... but they add up. If we now look at the enthalpies of covalent bonds, it is obvious that entropy does not play a significant role in the formation of bonds. Example: Reaction of two molecules forming one new C-C bond: ΔG = - 415 KJ/mol + 23 KJ/mol. The entropic effect accounts for less than 5% of the ...
... but they add up. If we now look at the enthalpies of covalent bonds, it is obvious that entropy does not play a significant role in the formation of bonds. Example: Reaction of two molecules forming one new C-C bond: ΔG = - 415 KJ/mol + 23 KJ/mol. The entropic effect accounts for less than 5% of the ...
6 theoretical problems 2 practical problems
... 1) empirical formula Pt(NH3)2Cl2, 2) an anion and a cation and is composed of discrete, monomeric square planar platinum(II) complex, 3) only one type of cation and one type of anion. The answer must clearly reveal the composition of each discrete platinum(II) complex entity in each compound ...
... 1) empirical formula Pt(NH3)2Cl2, 2) an anion and a cation and is composed of discrete, monomeric square planar platinum(II) complex, 3) only one type of cation and one type of anion. The answer must clearly reveal the composition of each discrete platinum(II) complex entity in each compound ...
000217986-Tajbakhsh_et_al_
... M. Tajbakhsh,a,* M. M. Lakouraj,a,* F. Shirini,b S. Habibzadeha and A. Nikdoosta a ...
... M. Tajbakhsh,a,* M. M. Lakouraj,a,* F. Shirini,b S. Habibzadeha and A. Nikdoosta a ...
SYLLABUS 5070 Cambridge O Level Chemistry
... Developed for an international audience Cambridge O Levels have been designed for an international audience and are sensitive to the needs of different countries. These qualifications are designed for students whose first language may not be English and this is acknowledged throughout the examinatio ...
... Developed for an international audience Cambridge O Levels have been designed for an international audience and are sensitive to the needs of different countries. These qualifications are designed for students whose first language may not be English and this is acknowledged throughout the examinatio ...
Empirical and Molecular Formulas
... Succinic acid is a substance produced by lichens. Chemical analysis indicates it is composed of 40.68% C, 5.08% H, and 54.24% oxygen and has a molar mass of 118.1g/mol. Determine the empirical formula for succinic acid. Step 1: Determine molar mass. 1st: Convert percentages into grams of elements. 2 ...
... Succinic acid is a substance produced by lichens. Chemical analysis indicates it is composed of 40.68% C, 5.08% H, and 54.24% oxygen and has a molar mass of 118.1g/mol. Determine the empirical formula for succinic acid. Step 1: Determine molar mass. 1st: Convert percentages into grams of elements. 2 ...
Microsoft Word - Open Access Repository of Indian Theses
... optimized by size and shape considerations. There are approximately 40 naturally occurring and over 100 synthetic forms of zeolites. The primary building blocks of the zeolites are the (SiO 4)4- ...
... optimized by size and shape considerations. There are approximately 40 naturally occurring and over 100 synthetic forms of zeolites. The primary building blocks of the zeolites are the (SiO 4)4- ...
Learning Guide for Chapter 9 - Alkyl Halides I
... This chapter will focus on: alkyl halides Compounds with more than one halide can be classified by how close together the halides are. Which of these is a geminal dihalide, and which is a vicinal dihalide? these are special kinds of dihalides Cl ...
... This chapter will focus on: alkyl halides Compounds with more than one halide can be classified by how close together the halides are. Which of these is a geminal dihalide, and which is a vicinal dihalide? these are special kinds of dihalides Cl ...
Chapter 3
... • identify the longest chain to which the ketone/aldehyde is attached, name it, append suffix “one”/“al” • indicate the position of the ketone on the chain with number (aldehyde is always terminal, no number) • if multiple ketones/aldehydes attached to same chain, use number prefix (di, tri, tetra) • ...
... • identify the longest chain to which the ketone/aldehyde is attached, name it, append suffix “one”/“al” • indicate the position of the ketone on the chain with number (aldehyde is always terminal, no number) • if multiple ketones/aldehydes attached to same chain, use number prefix (di, tri, tetra) • ...
File - Junior College Chemistry tuition
... hydrogen atom on the benzene ring but not for a hydrogen atom on the alkyl branches or in the –OH group. Given that any number of the benzene hydrogen may be substituted, how many possible products of the reaction are there? A ...
... hydrogen atom on the benzene ring but not for a hydrogen atom on the alkyl branches or in the –OH group. Given that any number of the benzene hydrogen may be substituted, how many possible products of the reaction are there? A ...
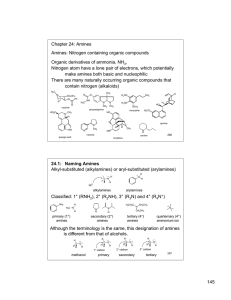
145 Chapter 24: Amines Amines: Nitrogen containing organic
... Symmetrical secondary and tertiary amines are named by adding the prefix di- or tri- to the alkyl group Unsymmetrical secondary and tertiary amines are named as N-substituted primary amines. The largest alkyl group is the parent name, and other alkyl groups are considered N-substituents. H H3CH2C ...
... Symmetrical secondary and tertiary amines are named by adding the prefix di- or tri- to the alkyl group Unsymmetrical secondary and tertiary amines are named as N-substituted primary amines. The largest alkyl group is the parent name, and other alkyl groups are considered N-substituents. H H3CH2C ...
Chapter 1 Chirality in clinical analysis 1.1. Introduction
... pharmacological effects, which may relate to their stereoselective pharmacokinetics or pharmacodynamics. The terms “eutomer” for the more potent isomer and “distomer” for the less potent one have been suggested [33]. The differences in the enantiomer pharmacodynamic activity and pharmacokinetic prop ...
... pharmacological effects, which may relate to their stereoselective pharmacokinetics or pharmacodynamics. The terms “eutomer” for the more potent isomer and “distomer” for the less potent one have been suggested [33]. The differences in the enantiomer pharmacodynamic activity and pharmacokinetic prop ...
Calculations on the equations reaction
... Determine the degree of oxidation of elements. Write the oxidant and reductant. 20.To equalize the oxidation-reduction reaction by electron balance method KMnO4 + HCl → MnCl2 + H2O +Cl2 + KCl . Determine the degree of oxidation of elements. Write the oxidant and reductant. Calculations based on the ...
... Determine the degree of oxidation of elements. Write the oxidant and reductant. 20.To equalize the oxidation-reduction reaction by electron balance method KMnO4 + HCl → MnCl2 + H2O +Cl2 + KCl . Determine the degree of oxidation of elements. Write the oxidant and reductant. Calculations based on the ...
HMDS - Sigma
... phases include SPB™-1 and SPB-5. Normal hydrocarbons (carbonhydrogen analytes with single bonds) are separated by these phases. More polar phases, SPB-1701 and SP™-2250, separate carbon-hydrogen analytes that also contain Br, Cl, F, N, O, P, or S atoms or groups. A highly polar cyanopropylphenylsilo ...
... phases include SPB™-1 and SPB-5. Normal hydrocarbons (carbonhydrogen analytes with single bonds) are separated by these phases. More polar phases, SPB-1701 and SP™-2250, separate carbon-hydrogen analytes that also contain Br, Cl, F, N, O, P, or S atoms or groups. A highly polar cyanopropylphenylsilo ...
Chapter 20 Amines - FIU Faculty Websites
... The acyl azide is obtained from an acid chloride Rearrangement of the acyl azide occurs with loss of N2, a very stable leaving group In the last step, the isocyanate is hydrolyzed by adding water ...
... The acyl azide is obtained from an acid chloride Rearrangement of the acyl azide occurs with loss of N2, a very stable leaving group In the last step, the isocyanate is hydrolyzed by adding water ...
Here
... (e) Gibbs free energy, G = H − TS, combines enthalpy and entropy to give a quantity which must decrease for any processes that actually happens. (f) Lewisite is a chlorinate alkyl arsenic compound which was produced as a chemical weapon causing blisters and lung irritation. (g) A Lewis base ...
... (e) Gibbs free energy, G = H − TS, combines enthalpy and entropy to give a quantity which must decrease for any processes that actually happens. (f) Lewisite is a chlorinate alkyl arsenic compound which was produced as a chemical weapon causing blisters and lung irritation. (g) A Lewis base ...
Chapter 1 – Reaction Kinetics Answer Key
... 3. The concentrations of pure solids and liquids are fixed. That is they do not change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. ...
... 3. The concentrations of pure solids and liquids are fixed. That is they do not change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. ...
1. What energy changes occur when chemical bonds are formed
... Separate solutions of HCl(aq) and H2SO4(aq) of the same concentration and same volume were completely neutralized by NaOH(aq). X kJ and Y kJ of heat were evolved respectively. Which statement is correct? A. ...
... Separate solutions of HCl(aq) and H2SO4(aq) of the same concentration and same volume were completely neutralized by NaOH(aq). X kJ and Y kJ of heat were evolved respectively. Which statement is correct? A. ...
CHEM 494 Lecture 10b - UIC Department of Chemistry
... Meissner 1819: To me it seems wholly appropriate to refer to those plant substances currently known by the names of alkalis, but alkaloids, since some of their properties they differ from alkalis considerably, and would thus find their place before the plant acids in the field of plant chemistry. Ja ...
... Meissner 1819: To me it seems wholly appropriate to refer to those plant substances currently known by the names of alkalis, but alkaloids, since some of their properties they differ from alkalis considerably, and would thus find their place before the plant acids in the field of plant chemistry. Ja ...
Unit 13: Organic Chemistry
... Regents Practice Problems-Carbon Compounds (ungraded): 1) Which compound must be present in an organic compound? ...
... Regents Practice Problems-Carbon Compounds (ungraded): 1) Which compound must be present in an organic compound? ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.