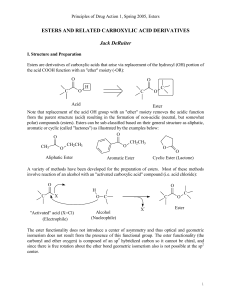
Iridium(III) and Rhodium(III) compounds of dipyridyl-N
... peaks at m/z = 470 and 435 due to ([(η5 -C5 Me5 )Rh {(C5 H4 N)2 C=N-Me}Cl])+ ([2]PF6 -PF6 )+ and ([(η5 C5 Me5 )Rh{(C5 H4 N)2 C=N-Me}])+ ([2]PF6 -PF6 -Cl)+ (see Supplementary Information). In addition, the spectrum also showed peak at m/z 421 due to ([(η5 -C5 Me5 ) Rh{(C5 H4 N)2 C=NH}])+ ([M]-PF6 -Cl ...
... peaks at m/z = 470 and 435 due to ([(η5 -C5 Me5 )Rh {(C5 H4 N)2 C=N-Me}Cl])+ ([2]PF6 -PF6 )+ and ([(η5 C5 Me5 )Rh{(C5 H4 N)2 C=N-Me}])+ ([2]PF6 -PF6 -Cl)+ (see Supplementary Information). In addition, the spectrum also showed peak at m/z 421 due to ([(η5 -C5 Me5 ) Rh{(C5 H4 N)2 C=NH}])+ ([M]-PF6 -Cl ...
CH 2 CH(CH 3 ) - Parkway C-2
... Always include both the double and triple bond in the longest chain – even if it isn’t the most number of carbons! Start counting from the end closest to the double or triple bond – whichever has the lowest number If there is a tie, and only then, do double bonds take priority over triple ...
... Always include both the double and triple bond in the longest chain – even if it isn’t the most number of carbons! Start counting from the end closest to the double or triple bond – whichever has the lowest number If there is a tie, and only then, do double bonds take priority over triple ...
Revised organic chemistry
... It has been found that isopropyl bromide is the major product. In such cases addition is governed by Markonikov’s rule which states that, “When an unsymmetrical reagent(such as HX, H2SO4,HOCl etc) adds to an unsymmetrical alkene, then the negative part of the reagent is added to that carbon atom of ...
... It has been found that isopropyl bromide is the major product. In such cases addition is governed by Markonikov’s rule which states that, “When an unsymmetrical reagent(such as HX, H2SO4,HOCl etc) adds to an unsymmetrical alkene, then the negative part of the reagent is added to that carbon atom of ...
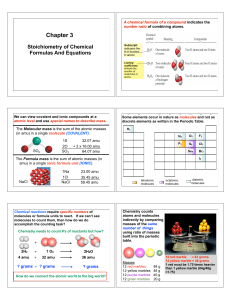
Chapter 3 2013
... 3. The molecular formula will be a whole number multiple of the empirical formula determined BY THE MOLAR MASS ratio ...
... 3. The molecular formula will be a whole number multiple of the empirical formula determined BY THE MOLAR MASS ratio ...
CHM 103 Lecture 24 S07
... • are carbon compounds that contain a –SH group. • are named in the IUPAC system by adding thiol to the alkane name of the longest carbon chain. • the -SH group may also be called a “mercapto” group ...
... • are carbon compounds that contain a –SH group. • are named in the IUPAC system by adding thiol to the alkane name of the longest carbon chain. • the -SH group may also be called a “mercapto” group ...
Worked solutions to the problems
... Ni(II), Cu(I), Cu(II), Ag(I), Zn(II), Hg(I), Hg(II) colors of the listed common ions in aqueous solution other oxidation stales and chemistry of other d-block elements Cr, Mn, Fe, Ni, Co, Zn dissolve in dil HCI; Cu, Ag, Hg do not dissolve products of the dissolution are (2+) cations passivation of C ...
... Ni(II), Cu(I), Cu(II), Ag(I), Zn(II), Hg(I), Hg(II) colors of the listed common ions in aqueous solution other oxidation stales and chemistry of other d-block elements Cr, Mn, Fe, Ni, Co, Zn dissolve in dil HCI; Cu, Ag, Hg do not dissolve products of the dissolution are (2+) cations passivation of C ...
Chapter 4: Types of Chemical Reactions and Solution Stoichiometry
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
... Example: If a solution containing potassium chloride is added to a solution containing ammonium nitrate, will a precipitate form? KCl(aq) + NH4NO3(aq) → K+(aq) + Cl-(aq) + NH4+(aq) + NO3-(aq) Possible reaction products are KCl and NH4NO3, NH4Cl and KNO3. All are soluble, so there is no precipitate. ...
Unit 5 Organic Chemistry
... Na2CO3(s), NaCN(s), and SiC(s), respectively. In spite of this broader definition, the major source of carbon compounds is still living or previously living things, such as plants, animals, and all types of fossil fuels. Coal, oil sands, heavy oil, crude oil, and natural gas are nonrenewable sources ...
... Na2CO3(s), NaCN(s), and SiC(s), respectively. In spite of this broader definition, the major source of carbon compounds is still living or previously living things, such as plants, animals, and all types of fossil fuels. Coal, oil sands, heavy oil, crude oil, and natural gas are nonrenewable sources ...
Disproportionation of Gold(II)
... The chemistry of gold is dominated by the chemistry of the monovalent (AuI) and trivalent (AuIII) ions. The former tend to be linear, two-coordinate complexes, whereas the latter are typically square planar, four-coordinate complexes. The chemistry of the intermediate formal oxidation state, divalen ...
... The chemistry of gold is dominated by the chemistry of the monovalent (AuI) and trivalent (AuIII) ions. The former tend to be linear, two-coordinate complexes, whereas the latter are typically square planar, four-coordinate complexes. The chemistry of the intermediate formal oxidation state, divalen ...
Organic Chemistry Fifth Edition
... transition state and an unstable intermediate) are similar in energy, they are similar in structure. Hammond's postulate permits us to infer the structure of something we can't study (transition state) from something we can study ...
... transition state and an unstable intermediate) are similar in energy, they are similar in structure. Hammond's postulate permits us to infer the structure of something we can't study (transition state) from something we can study ...
Chemistry 162 Workbook 10.6
... Cis/ Trans Please identify if the following are cis (with a circle) or trans (with a square) compounds. If the compound is neither cis or trans, leave it blank. ...
... Cis/ Trans Please identify if the following are cis (with a circle) or trans (with a square) compounds. If the compound is neither cis or trans, leave it blank. ...
- Catalyst
... Problem: The hydrocarbon hexane is a component of gasoline that burns in an automobile engine to produce carbon dioxide and water, as well as energy. Write the balanced chemical equation for the combustion of hexane (C6H14). Plan: Write the skeleton equation, converting the words into compounds, wit ...
... Problem: The hydrocarbon hexane is a component of gasoline that burns in an automobile engine to produce carbon dioxide and water, as well as energy. Write the balanced chemical equation for the combustion of hexane (C6H14). Plan: Write the skeleton equation, converting the words into compounds, wit ...
Stoichiometry - AaronFreeman
... Mesitylene, a hydrocarbon that occurs in small amounts in crude oil, has an empirical formula of C3H4. The experimentally determined molecular weight of this substance is 121 amu. What is the molecular formula of mesitylene? 1. Calculate the empirical formula weight of C3H4 ...
... Mesitylene, a hydrocarbon that occurs in small amounts in crude oil, has an empirical formula of C3H4. The experimentally determined molecular weight of this substance is 121 amu. What is the molecular formula of mesitylene? 1. Calculate the empirical formula weight of C3H4 ...
An Introduction to Chemical Science
... observations to be made, etc. The right-hand page is blank, and on that the pupil makes a record of his work. These notes are examined at the time, or subsequently, by the teacher, and the pupil is not allowed to take the book from the laboratory; nor can he use any other book on Chemistry while exp ...
... observations to be made, etc. The right-hand page is blank, and on that the pupil makes a record of his work. These notes are examined at the time, or subsequently, by the teacher, and the pupil is not allowed to take the book from the laboratory; nor can he use any other book on Chemistry while exp ...
DEPARTMENT OF CHEMISTRY, UNIVERSITY OF JYVÄSKYLÄ
... the formation of molecules with great accuracy, less is still known about what the molecules can do with their bonds. The reactivity of low-valent main group compounds is a primary example: at first, these species were thought to be inert in reactions toward small molecules, such as hydrogen and ole ...
... the formation of molecules with great accuracy, less is still known about what the molecules can do with their bonds. The reactivity of low-valent main group compounds is a primary example: at first, these species were thought to be inert in reactions toward small molecules, such as hydrogen and ole ...
Penny Commons - Chemistry Education Association
... The chapter numbers refer to the new 5th Edition Pearson Heinemann Chemistry 2 – both in the print version and the fully electronic and interactive Pearson Lightbook Chemistry Victoria 21. The pracs, exercises and demonstrations are all found in old editions of Pearson Heinemann TRAB or in the S ...
... The chapter numbers refer to the new 5th Edition Pearson Heinemann Chemistry 2 – both in the print version and the fully electronic and interactive Pearson Lightbook Chemistry Victoria 21. The pracs, exercises and demonstrations are all found in old editions of Pearson Heinemann TRAB or in the S ...
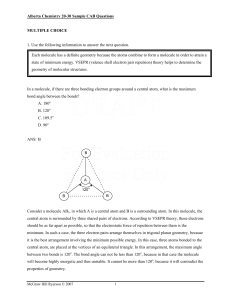
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...
Organic Chemistry
... [1,2]. π-Conjugated materials are extensively investigated and explored for OLEDs because of their potential in the creation of cost-effective, power-efficient, and flexible electronic devices [35]. Nowadays aromatic cores with a large π-orbital area are used as hole-transporting materials. These mole ...
... [1,2]. π-Conjugated materials are extensively investigated and explored for OLEDs because of their potential in the creation of cost-effective, power-efficient, and flexible electronic devices [35]. Nowadays aromatic cores with a large π-orbital area are used as hole-transporting materials. These mole ...
mole ratio
... How to Work Stoichiometry Problems 1. Write and balance the equation. 2. Convert mass or volume or particles to moles, if necessary. 3. Set up mole ratios and calculate moles of desired component (product or reactant). 4. Convert moles to mass or volume or ...
... How to Work Stoichiometry Problems 1. Write and balance the equation. 2. Convert mass or volume or particles to moles, if necessary. 3. Set up mole ratios and calculate moles of desired component (product or reactant). 4. Convert moles to mass or volume or ...
guess paper class xii
... 16 In a fuel cell (a device for producing electricity directly from chemical reaction) , methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is: CH3OH (l) + 3/2 O2 CO2 (g) + 2H2O (l) Calculate the standard Gibbs energy change for the reaction that can be converted into elect ...
... 16 In a fuel cell (a device for producing electricity directly from chemical reaction) , methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is: CH3OH (l) + 3/2 O2 CO2 (g) + 2H2O (l) Calculate the standard Gibbs energy change for the reaction that can be converted into elect ...
Brilliant Preparatory Section, Sitamarhi
... products. The skeletal equation is KMnO4 + HCl → KCl + MnCl2 + H2O + Cl2 If an element is present only one substance in the left hand side of the equation and if the same element is present only one of the substances in the right side, it may be taken up first while balancing the equation. According ...
... products. The skeletal equation is KMnO4 + HCl → KCl + MnCl2 + H2O + Cl2 If an element is present only one substance in the left hand side of the equation and if the same element is present only one of the substances in the right side, it may be taken up first while balancing the equation. According ...
tro2_ppt_lecture_04 - Louisiana Tech University
... 6 CO2(g) + 6 H2O(l) C6H6O6(s) + 6 O2(g) 2. Determine moles of CO2 consumed. 37.8 g CO2 x (1 mol CO2/44.0 g) = 0.859 mol CO2 3. Determine how many moles of C6H6O6 would be produced. Use the stoichiometric relationship between CO2 and C6H6O6. In this reaction the relationship is 6 mole CO2 to 1 mole ...
... 6 CO2(g) + 6 H2O(l) C6H6O6(s) + 6 O2(g) 2. Determine moles of CO2 consumed. 37.8 g CO2 x (1 mol CO2/44.0 g) = 0.859 mol CO2 3. Determine how many moles of C6H6O6 would be produced. Use the stoichiometric relationship between CO2 and C6H6O6. In this reaction the relationship is 6 mole CO2 to 1 mole ...
organic families
... b Carefully smell the liquid. c Place 1 mL of the liquid in a test tube. Add 1 mL of sulfuric acid solution and 10 drops of potassium dichromate solution. Stand the test tube in a hot water bath for two minutes. Note You are required to wear safety glasses and the contents of your test tubes are to ...
... b Carefully smell the liquid. c Place 1 mL of the liquid in a test tube. Add 1 mL of sulfuric acid solution and 10 drops of potassium dichromate solution. Stand the test tube in a hot water bath for two minutes. Note You are required to wear safety glasses and the contents of your test tubes are to ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.