Exercises Chem Eqm
... 7.1(a) K = 2.85 x 10-6; (b) ∆rGo = +240 kJ mol-1; (c) ∆rG = 0 7.4(a) Mole fractions A: 0.087, B: 0.370, C: 0.196, D: 0.348, Total: 1.001; (b) Kx – 0.33; (c) p = 0.33; (d) ∆rGo = + 2.8 x 103 J mol-1. 7.6(a) ∆rHo = +2.77 kJ mol-1, ∆rSo = -16.5 J K-1 mol-1 7.9(a) χB = 0.904, χI = 0.096 7.11(a) ∆rGo = – ...
... 7.1(a) K = 2.85 x 10-6; (b) ∆rGo = +240 kJ mol-1; (c) ∆rG = 0 7.4(a) Mole fractions A: 0.087, B: 0.370, C: 0.196, D: 0.348, Total: 1.001; (b) Kx – 0.33; (c) p = 0.33; (d) ∆rGo = + 2.8 x 103 J mol-1. 7.6(a) ∆rHo = +2.77 kJ mol-1, ∆rSo = -16.5 J K-1 mol-1 7.9(a) χB = 0.904, χI = 0.096 7.11(a) ∆rGo = – ...
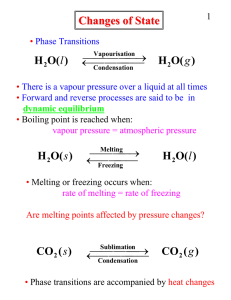
Liquid-gas phase change dynamic equilibrium: forward and reverse
... increase in enthalpy (ΔH > 0) of the substance because the molecules need to gain energy to overcome the IMF’s. endothermic: a process in which the system gains energy. ΔH > 0 In vaporization, melting, or sublimation, a substance absorbs energy, allowing the molecules to overcome intermolecular attr ...
... increase in enthalpy (ΔH > 0) of the substance because the molecules need to gain energy to overcome the IMF’s. endothermic: a process in which the system gains energy. ΔH > 0 In vaporization, melting, or sublimation, a substance absorbs energy, allowing the molecules to overcome intermolecular attr ...
Introduction to Thermodynamics I. Conservation of Energy
... f) Example: Predict the sign of the entropy change for i) Dissolving solid sugar into water ii) Iodine vapor condensing to crystals on a surface 7) Second Law of Thermodynamics = in any spontaneous process, there is always an increase in the entropy of the universe a) Energy is conserved = constant ...
... f) Example: Predict the sign of the entropy change for i) Dissolving solid sugar into water ii) Iodine vapor condensing to crystals on a surface 7) Second Law of Thermodynamics = in any spontaneous process, there is always an increase in the entropy of the universe a) Energy is conserved = constant ...
Introduction to the Chemistry of Life
... The first law of thermodynamics states that energy is conserved and can be neither created nor destroyed. It may, however, be transferred, for example, in the form of heat or work, between a closed or open system and its surroundings. The second law of thermodynamics states that spontaneous processe ...
... The first law of thermodynamics states that energy is conserved and can be neither created nor destroyed. It may, however, be transferred, for example, in the form of heat or work, between a closed or open system and its surroundings. The second law of thermodynamics states that spontaneous processe ...
Frenkel and Smit / Chandler
... inducing a dipole in the molecule in a manner such that the induced dipole is proportional (linearly related) to the applied field strength. The proportionality constant is called the polarizability of the molecule. ...
... inducing a dipole in the molecule in a manner such that the induced dipole is proportional (linearly related) to the applied field strength. The proportionality constant is called the polarizability of the molecule. ...
GRE-thermo
... A car of rest length 5 meters passes through a garage of rest length 4 meters. Due to the relativistic Lorentz contraction, the car is only 3 meters long in the garage's rest frame. There are doors on both ends of the garage, which open automatically when the front of the car reaches them and close ...
... A car of rest length 5 meters passes through a garage of rest length 4 meters. Due to the relativistic Lorentz contraction, the car is only 3 meters long in the garage's rest frame. There are doors on both ends of the garage, which open automatically when the front of the car reaches them and close ...
Introduction to Physical Chemistry
... Overview: This class will introduce concepts of physical chemistry particularly relevant to the medicinal and life sciences. While developing the tools to solve quantitative problems in the physical sciences is an integral part of this class, an emphasis will be placed on developing a deeper concept ...
... Overview: This class will introduce concepts of physical chemistry particularly relevant to the medicinal and life sciences. While developing the tools to solve quantitative problems in the physical sciences is an integral part of this class, an emphasis will be placed on developing a deeper concept ...
Faculty of Science Department of chemistry Practical Physical
... Course Description: The Experiments: We will perform at least 12 experiments in physical chemistry covering Thermodynamics and Equilibrium. Students in the laboratory course will rotate through the list of experiments in 2 groups, designated I and II to best utilize our available equipments. You wil ...
... Course Description: The Experiments: We will perform at least 12 experiments in physical chemistry covering Thermodynamics and Equilibrium. Students in the laboratory course will rotate through the list of experiments in 2 groups, designated I and II to best utilize our available equipments. You wil ...
Study Guide for Final Exam
... control volume, which for a fixed mass is a control mass. Such a system can be isolated, exchanging neither mass, momentum, nor energy with its surroundings. A closed system versus an open system refers to the ability of mass exchange with the surroundings. If properties for a substance change, the ...
... control volume, which for a fixed mass is a control mass. Such a system can be isolated, exchanging neither mass, momentum, nor energy with its surroundings. A closed system versus an open system refers to the ability of mass exchange with the surroundings. If properties for a substance change, the ...
Chapter 1. The Birth of Modern Physics
... constraint on how energy can be transformed from one form into another. For example, although it is clear that an amount of work can be transformed into heat, can this heat be afterwards transform back into work? This question is addressed with the Second Law, which can be stated as follows (there a ...
... constraint on how energy can be transformed from one form into another. For example, although it is clear that an amount of work can be transformed into heat, can this heat be afterwards transform back into work? This question is addressed with the Second Law, which can be stated as follows (there a ...
Lecture 4. - ChemWeb (UCC)
... • Energy associated with random motion of atoms and molecules Chemical Energy • Energy stored in structural units of chemicals Units = Joules • 1 Joule (J) = 1 kg m2 /s2 • 1 Calorie (cal) = 4.184 J ...
... • Energy associated with random motion of atoms and molecules Chemical Energy • Energy stored in structural units of chemicals Units = Joules • 1 Joule (J) = 1 kg m2 /s2 • 1 Calorie (cal) = 4.184 J ...
Origin of Order: Emergence and Evolution of Biological Organization
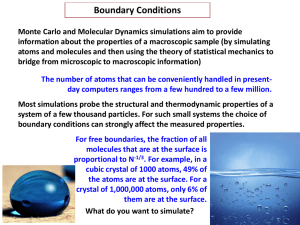
... dissipation of thermal energy (heat) and the interrelation between heat and other forms of energy. Thermodynamics is also concerned with systems of very large numbers of particles so that thermodynamic variables, such as pressure, volume, and temperature, are considered as statistical quantities. Th ...
... dissipation of thermal energy (heat) and the interrelation between heat and other forms of energy. Thermodynamics is also concerned with systems of very large numbers of particles so that thermodynamic variables, such as pressure, volume, and temperature, are considered as statistical quantities. Th ...
Gibbs Free Energy and chemical equilibrium
... • We want to answer these questions: • Will this reaction go? • If so, how far can it proceed? We will do this by using thermodynamics. • This lecture will be restricted to a small subset of thermodynamics... ...
... • We want to answer these questions: • Will this reaction go? • If so, how far can it proceed? We will do this by using thermodynamics. • This lecture will be restricted to a small subset of thermodynamics... ...
Chapter 19 Chemical Thermodynamics
... • Entropy can be thought of as a measure of the randomness of a system. • It is related to the various modes of motion in molecules. ...
... • Entropy can be thought of as a measure of the randomness of a system. • It is related to the various modes of motion in molecules. ...
Physical Chemistry
... course covers classical thermodynamics and chemical kinetics. Detailed treatment of classical thermodynamics and application to chemical phenomena in macroscopic systems is introduced. Topics include properties of gases, equations of state, laws of thermodynamics, phase and reaction equilibrium, the ...
... course covers classical thermodynamics and chemical kinetics. Detailed treatment of classical thermodynamics and application to chemical phenomena in macroscopic systems is introduced. Topics include properties of gases, equations of state, laws of thermodynamics, phase and reaction equilibrium, the ...
CCN2275 Physical Chemistry
... general knowledge obtained in lectures. Problem-based activities, classroom feedback, and discussions will be arranged in tutorials to stimulate students’ interest or their awareness of the practical implications of some concepts. Laboratory sessions may also be used to enable students to understand ...
... general knowledge obtained in lectures. Problem-based activities, classroom feedback, and discussions will be arranged in tutorials to stimulate students’ interest or their awareness of the practical implications of some concepts. Laboratory sessions may also be used to enable students to understand ...
Chemical Equilibrium
... probability of there being any significant excitation at temperatures of interest. (This is not so for large molecules and solids.) A large energy gap means a large Qelec and, assuming that T << Qelec, we have CVelec 0. Putting these contributions to the heat capacity of a gas of H2 together we ha ...
... probability of there being any significant excitation at temperatures of interest. (This is not so for large molecules and solids.) A large energy gap means a large Qelec and, assuming that T << Qelec, we have CVelec 0. Putting these contributions to the heat capacity of a gas of H2 together we ha ...
College application essay about vignette
... The force that binds protons and neutrons together in the atomic nucleus. Sublimation The process by which a solid turns directly into gas, because it cannot exist as a liquid at a certain pressure. Superposition The principle by which the displacements from different waves traveling in the same med ...
... The force that binds protons and neutrons together in the atomic nucleus. Sublimation The process by which a solid turns directly into gas, because it cannot exist as a liquid at a certain pressure. Superposition The principle by which the displacements from different waves traveling in the same med ...