Chapter 6 lecture 1
... All objects above 0 K emit radiation Objects at around room temperature emit mainly infrared radiation (» 10mm) which is invisible. The sun emits most of its radiation at visible wavelengths, particularly yellow ( » 0.5 mm) The maximum wavelength of radiation emitted depends on temperature: ...
... All objects above 0 K emit radiation Objects at around room temperature emit mainly infrared radiation (» 10mm) which is invisible. The sun emits most of its radiation at visible wavelengths, particularly yellow ( » 0.5 mm) The maximum wavelength of radiation emitted depends on temperature: ...
Quantum theory or radiation
... In 1900 the problem was solved in a revolutionary way by a professor from Berlin University, Max Planck. Planck was born in 1858 in Keil, was educated in Munich and Berlin, becoming a professor in Keil in 1885 before moving to Berlin in 1889. He described his radiation formula as "lucky" but it invo ...
... In 1900 the problem was solved in a revolutionary way by a professor from Berlin University, Max Planck. Planck was born in 1858 in Keil, was educated in Munich and Berlin, becoming a professor in Keil in 1885 before moving to Berlin in 1889. He described his radiation formula as "lucky" but it invo ...
Light problems
... 8.____ A quantum of energy is the a. frequency of electromagnetic energy given off by an atom. b. wavelength of electromagnetic energy gained by an atom. c. minimum quantity of energy that can be lost or gained by an atom. d. continuous spectrum of energy given off by an atom. 9.____ A form of energ ...
... 8.____ A quantum of energy is the a. frequency of electromagnetic energy given off by an atom. b. wavelength of electromagnetic energy gained by an atom. c. minimum quantity of energy that can be lost or gained by an atom. d. continuous spectrum of energy given off by an atom. 9.____ A form of energ ...
Elec Structure of Atom
... carries energy through space – it moves through a vacuum at the speed of light (3 x 108 m/s). ...
... carries energy through space – it moves through a vacuum at the speed of light (3 x 108 m/s). ...
Information in Radio Waves
... __________________________________________________________ __________________________________________________________ ...
... __________________________________________________________ __________________________________________________________ ...
Max Planck suggested that the energy of light is proportional to its
... properties; visible light being a well-known example. From the wave perspective, all forms of EM radiation may be described in terms of their wavelength and frequency. Wavelength is the distance from one wave peak to the next, which can be measured in meters. Frequency is the number of waves that pa ...
... properties; visible light being a well-known example. From the wave perspective, all forms of EM radiation may be described in terms of their wavelength and frequency. Wavelength is the distance from one wave peak to the next, which can be measured in meters. Frequency is the number of waves that pa ...
1 – Foundations of Quantum Theory
... • Classical theory views light as a wave • Some argue that the father of Quantum theory was Einstein because Planck didn’t really realize what the math said (he kept trying to make light a wave when his calculations seem to refer to a packet of energy whose size varies with frequency) ...
... • Classical theory views light as a wave • Some argue that the father of Quantum theory was Einstein because Planck didn’t really realize what the math said (he kept trying to make light a wave when his calculations seem to refer to a packet of energy whose size varies with frequency) ...
03 Starlight and Atoms
... (where F (flux) is power/unit area, σ = 5.67 x 10-8 W/m2·K4 , TK is the temperature in Kelvin). 2. The peak of the black body spectrum shifts towards shorter wavelengths when the temperature increases. Wien’s displacement law: ...
... (where F (flux) is power/unit area, σ = 5.67 x 10-8 W/m2·K4 , TK is the temperature in Kelvin). 2. The peak of the black body spectrum shifts towards shorter wavelengths when the temperature increases. Wien’s displacement law: ...
Introduction to Spectroscopy
... the index of refraction with frequency, leading to phase changes in the light that are frequency dependent ...
... the index of refraction with frequency, leading to phase changes in the light that are frequency dependent ...
Document
... The intensity of the wave (represented by a wave function at a point in space represents the probability of observing a particle at that location. ...
... The intensity of the wave (represented by a wave function at a point in space represents the probability of observing a particle at that location. ...
Lecture 24: Quantum mechanics
... Non-classical Explanation: Planck hypothesis. Planck assumed that the radiating substance was composed of electric dipoles that acted as simple harmonic oscillators. His suggestion was as follows: The energy of an oscillator must be discrete. E = n h where n is an integer, h is a constant of propo ...
... Non-classical Explanation: Planck hypothesis. Planck assumed that the radiating substance was composed of electric dipoles that acted as simple harmonic oscillators. His suggestion was as follows: The energy of an oscillator must be discrete. E = n h where n is an integer, h is a constant of propo ...
Set 3
... 4) The wavelength of X-ray radiation equals to 35 pm. What is the corresponding frequency? Please calculate the energy of the photon and its momentum. 5) In which range of the electromagnetic radiation the momentum of photons takes the largest value: ultraviolet, X-ray radiation, radio waves, infrar ...
... 4) The wavelength of X-ray radiation equals to 35 pm. What is the corresponding frequency? Please calculate the energy of the photon and its momentum. 5) In which range of the electromagnetic radiation the momentum of photons takes the largest value: ultraviolet, X-ray radiation, radio waves, infrar ...
Physics 120 Homework Set #1 (due Sunday
... b) Does the principle describe a property of a quantum object (e.g. electron) or the limitations of an action (i.e. observation or measurement)? Explain. The limit posed by the Uncertainty Principle is not a consequence of some deficiency in the experimental techniques. Rather, it signifies that an ...
... b) Does the principle describe a property of a quantum object (e.g. electron) or the limitations of an action (i.e. observation or measurement)? Explain. The limit posed by the Uncertainty Principle is not a consequence of some deficiency in the experimental techniques. Rather, it signifies that an ...
Lec-22_Strachan
... linear momentum is made with precision Δpx, then the product of the two uncertainties can never be smaller than h/4 ...
... linear momentum is made with precision Δpx, then the product of the two uncertainties can never be smaller than h/4 ...
For a “black body” - The University of Sheffield
... To explain results of the Rutherford scattering : 1) Atom must be mostly empty space 2) Positive charge must be concentrated in a small volume occupying a very small fraction of the total volume of the atom………… ...
... To explain results of the Rutherford scattering : 1) Atom must be mostly empty space 2) Positive charge must be concentrated in a small volume occupying a very small fraction of the total volume of the atom………… ...
Spectroscopy - Birmingham City Schools
... Spectra 1897 Max Karl Ernst Ludwig Planck proposed idea of quanta o certain energy = quanta of energy o E = h = hc h = Planck’s constant (6.63 x 10-34 J s), E = energy, = frequency = c c = speed of light (3 x 108 m/s) Since 1859 scientists using spectral lines to identify eleme ...
... Spectra 1897 Max Karl Ernst Ludwig Planck proposed idea of quanta o certain energy = quanta of energy o E = h = hc h = Planck’s constant (6.63 x 10-34 J s), E = energy, = frequency = c c = speed of light (3 x 108 m/s) Since 1859 scientists using spectral lines to identify eleme ...
Some quantum properties of light
... In order for one to achieve population inversion the lifetime of the metastable state must be greater than time atom spends in ground state or pump state during the pumping process. ...
... In order for one to achieve population inversion the lifetime of the metastable state must be greater than time atom spends in ground state or pump state during the pumping process. ...
SESSION 6: ELECTROMAGNETIC RADIATION KEY CONCEPTS: X
... Question 1: Calculate the frequency of an EM wave with a wavelength of 400 nm. Question 2: What is the wavelength of a photon of light with a frequency of 101.3 kHz? Question 3: Give an example of the use of each type of EM radiation, i.e. gamma rays, X-rays, ultraviolet light, visible light, infrar ...
... Question 1: Calculate the frequency of an EM wave with a wavelength of 400 nm. Question 2: What is the wavelength of a photon of light with a frequency of 101.3 kHz? Question 3: Give an example of the use of each type of EM radiation, i.e. gamma rays, X-rays, ultraviolet light, visible light, infrar ...
Pre-AP Chemistry
... Radiation and Spectra Review. Complete the following short answer questions. 1. What kind of proportion is shown by the equation c = λ ν? 2. Who stated that electromagnetic radiation has a dual nature? 3. How many meters are 665 nm equal to? 4. What wave measure is identified by the unit Hz? 5. What ...
... Radiation and Spectra Review. Complete the following short answer questions. 1. What kind of proportion is shown by the equation c = λ ν? 2. Who stated that electromagnetic radiation has a dual nature? 3. How many meters are 665 nm equal to? 4. What wave measure is identified by the unit Hz? 5. What ...
here
... Properties of thermal radiation Hotter objects emit more photons, so hotter objects are brighter objects Energy emitted per unit surface area ~ T4 Double an object’s temperature, and it emits 16 times as much energy! (16 = 24) Triple the temperature, and it emits 81 times as much energy!! ...
... Properties of thermal radiation Hotter objects emit more photons, so hotter objects are brighter objects Energy emitted per unit surface area ~ T4 Double an object’s temperature, and it emits 16 times as much energy! (16 = 24) Triple the temperature, and it emits 81 times as much energy!! ...
Quantum Physics 2 - More About
... prediction, first made in the late 19th century, that an IDEAL BLACK BODY at thermal equilibrium will emit radiation with INFINITE POWER. Max Planck resolved this issue by postulating that electromagnetic energy did not follow the classical description, but could only oscillate or be emitted in DISC ...
... prediction, first made in the late 19th century, that an IDEAL BLACK BODY at thermal equilibrium will emit radiation with INFINITE POWER. Max Planck resolved this issue by postulating that electromagnetic energy did not follow the classical description, but could only oscillate or be emitted in DISC ...
Chemistry Science Notebook
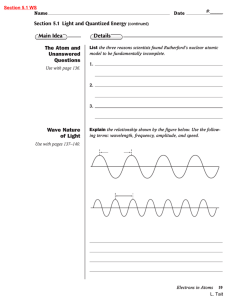
... Explain the relationship shown by the figure below. Use the following terms: wavelength, frequency, amplitude, and speed. ...
... Explain the relationship shown by the figure below. Use the following terms: wavelength, frequency, amplitude, and speed. ...