Branched-Chain Alkanes
... Aromatic Hydrocarbons – There is a class of unsaturated cyclic hydrocarbons that are responsible for the aromas of spices such as vanilla, cinnamon, cloves, and ginger. – These compounds were originally called aromatic compounds because they have distinct, pleasant aromas. – However not all compoun ...
... Aromatic Hydrocarbons – There is a class of unsaturated cyclic hydrocarbons that are responsible for the aromas of spices such as vanilla, cinnamon, cloves, and ginger. – These compounds were originally called aromatic compounds because they have distinct, pleasant aromas. – However not all compoun ...
Results
... Conformation D is sufficiently destabilized so only A, B and C are expected to show decarbonylation reaction The following 3 families of mechanisms may be anticipated A: Unimolecular Internal SN2 type reactionmechanism (SNi) with alactone formation B: Unimolecular Internal Addition/elimination mecha ...
... Conformation D is sufficiently destabilized so only A, B and C are expected to show decarbonylation reaction The following 3 families of mechanisms may be anticipated A: Unimolecular Internal SN2 type reactionmechanism (SNi) with alactone formation B: Unimolecular Internal Addition/elimination mecha ...
Pd presentation
... •Usually has high yield and selectivity •Less expensive than Platinum, which has similar properties Palladium can be used in a wide variety of reactions: •Stille- between organohalides & organotin compounds •Buchwald-Hartwig- between aryl halide & amine or aryl alcohol •Tsuji-Trost- between alkene & ...
... •Usually has high yield and selectivity •Less expensive than Platinum, which has similar properties Palladium can be used in a wide variety of reactions: •Stille- between organohalides & organotin compounds •Buchwald-Hartwig- between aryl halide & amine or aryl alcohol •Tsuji-Trost- between alkene & ...
Lecture notes for chapter 6
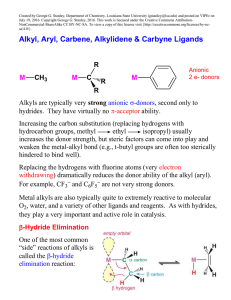
... Aryl ligands are relatively strong anionic two M electron donors, essentially just like alkyls. Since they cannot easily -hydride eliminate (formation of the benzyne intermediate is typically too unstable), metal aryl complexes are usually relatively stable compared to alkyls with hydrogens. But “ ...
... Aryl ligands are relatively strong anionic two M electron donors, essentially just like alkyls. Since they cannot easily -hydride eliminate (formation of the benzyne intermediate is typically too unstable), metal aryl complexes are usually relatively stable compared to alkyls with hydrogens. But “ ...
Summer Scholar Report
... (11). All glassware was dried and flushed with nitrogen. 1.558 g (0.010 moles) of lmenthol in 15 mL freshly dried THF was added to a 100 mL three-neck round bottom flask equipped with a condenser. 0.273 g (0.011 moles, excess) of sodium hydride was then added with 30 mL of THF, the reaction was heat ...
... (11). All glassware was dried and flushed with nitrogen. 1.558 g (0.010 moles) of lmenthol in 15 mL freshly dried THF was added to a 100 mL three-neck round bottom flask equipped with a condenser. 0.273 g (0.011 moles, excess) of sodium hydride was then added with 30 mL of THF, the reaction was heat ...